The process of speciation (multiplication of species) and its relation to the rate of morphological change are one of the most interesting and important subjects in evolutionary paleontology. Various theoretical models and valid new concepts have been put forward in recent years, but it may be said that positive approaches do not necessarily keep up with theoretical studies. This is, of course, primarily due to the inevitable incompleteness of fossil records. Nevertheless, models and theories should be tested in various actual taxonomic groups which are well represented both in fossils and present seas.
As Mayr (1954), Knox (1963), Fretter and Graham (1963) and some others have discussed, there is no reason to consider that the speciation of marine animals is fundamentally different in mechanism from that of terrestrial ones. Though the present data on the speciation of Cryptopecten are far from ideal, the origin of each species can be provisionally interpreted in accordance with the widely accepted theory of geographic (allopatric) speciation (Mayr, 1942, 1963; etc.).
There are four living species of Cryptopecten in the world; namely, C. bullatus in the central-west Pacific, C. nux in the Indo-Pacific, C. vesiculosus in the seas around Japan and C. phrygium in the Gulf of Mexico and adjacent seas (see Fig. 2). They are clearly distinct from one another, not only in morphological characters (e. g., size, shell convexity, outline, surface sculpture and number of radial ribs) but also in geographic and bathymetric distribution (see Figs. 2, 3). Dead shells of the first three species were collected together at a few stations around the southern part of the Japanese Islands, but available records of living specimens indicate that their bathymetric ranges are considerably different; the optimum depth seems to be about 200 meters or still larger in C. bullatus and C. phrygium, about 100 meters in C. vesiculosus, and as shallow as 30 to 50 meters in C. nux. I have not seen any individual suggestive of a hybrid.
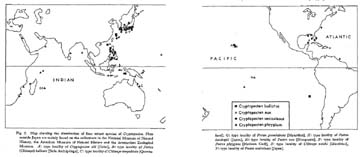
Fig. 2. Map showing the distribution of four extant species of Cryptopecten. Plots outside Japan are mainly based on the collections in the National Museum of Natural History, the American Museum of Natural History and the Amsterdam Zoological Museum. A: type locality of Cryptopecten alli [Oahu], B: type locality of Pecten (Chlamys) bullatus [Sulu Archipelago], C: type locality of Chlamys corymbiata [Queens-land], G: type locality of Pecten guendolenae [Mauritius], H: type locality of Pecten hastingsii [Japan], N: type locality of Pecten nux [Marquesas], P: type locality of Pecten phrygium [Mexican Gulf], S: type locality of Chlamys smithi [Mauritius], V: type locality of Pecten vesiculosus [Japan].
|

These three Indo-Pacific species seem to have evolved along independent lineages, at least since Middle Pliocene. In addition to the well-recognized stock of C. vesiculosus, the persistent lineage of C. nux with little morphological change with time is suggested by several Pliocene and Pleistocene samples in south Japan. Furthermore, according to Cox (1930) and Eames and Cox (1956), C. nux is a long-lived species from Early Miocene onward in east Africa. It is therefore probable that C. nux is the parental species from which some other species of Cryptopecten were derived. The lineage of C. bullatus, though its fossil records are still poor, is considered to have been established by the end of Middle Pliocene (Fig. 24).

So far as I am aware, no fossil record of C. phrygium has been known. It is a solitary Atlantic representative of this genus but seems to be closely related to C. bullatus in the Pacific. Not only their morphology but also their bathymetric ranges are so similar that
the two species seem to have a common ancestry predating the formation of the Isthmus of Panama. In fact, there are many pairs of vicarious molluscan species on the two sides of this land bridge. On the basis of DSDP cores from the Panamanian and Colombian sea basins, Keigwin (1978) supposed that the separation of the two water masses occurred in Middle Pliocene (about 3.1 Ma). Because C. bullatus has not been recorded along the western coast of the Americas (Grau, 1959, etc.), these two species of Cryptopecten may not constitute a typical case of vicarious species. However, as has been pointed out by many authors, the molluscan fauna of the Mexican Gulf and Caribbean Sea contains some elements closely related to the western Pacific fauna; for example, all of the three living genera (or subgenera) of the Pleurotomariidae, namely Perotrochus, Mikadotrochus and Entemnotrochus, are represented in each sea area respectively by one or a few species. C. phrygium was probably derived from C. bullatus by some geographic isolation, though the formation of the Isthmus of Panama may not be the direct cause of its speciation.
Needless to say, all the evidence indicating ancestral-descendant relation of fossil organisms is inevitably circumstantial. In the phylogenetic reconstruction of Cryptopecten, special stress is here laid upon the two different modes of imbricated scales on the sides of radial ribs in addition to the geographic and stratigraphic distribution of each species. C. bullatus and C. phrygium, as precisely described later, share similarly alternate disposition of imbricated scales with C. nux. Taking also some other characters into consideration, these three species seem to constitute an infrageneric species group. Though their fossil records are still very insufficient, C. bullatus may have been derived from C. nux through some geographic speciation and adapted to somewhat deeper substratum.
Incidentally, we know many pairs of vicarious (or closely related) molluscan species adapting to different bathymetric ranges in the same sea area. As to the origin and process of such marine vicarism, I think Diamond's model on the speciation of New Guinea birds (see MacArthur, 1972), in which two geographically isolated populations grow into vicariate species occupying different ranges of altitude in the mountainland, is quite suggestive.
C. vesiculosus, on the other hand, shares opposite disposition of imbricated scales with C. yanagawaensis from the Middle Miocene and C. spinosus from the Upper Pleistocene. This species group is known only from Japan and its surrounding seas, and the center of distribution in the geological ages is also expected to be in this region. As was interpreted by Masuda (1962), C. yanagawaensis is possibly ancestral to C. vesiculosus, though the wide gap of fossil records between Middle Miocene and Middle Pliocene makes the interspecific relation obscure. If this interpretation is correct, the number of radial ribs greatly decreased in this group.
In the course of this study I have noticed two aberrant fossil phena, which are certainly related to the stock of C. vesiculosus but morphologically different from other samples.
One is represented by the Late Pliocene samples Kg 1 and Kg 2 from the Hosoya Silt of the Kakegawa Group in central Honshu. The specimens belonging to these samples are characterized by the unusually large ultimate size, rapid growth rate, narrow central solid ridge of each rib, thin test and more numerous radial ribs on the disk (Plate 7, Figs. 11, 12). In the upper part of the Kakegawa Group all the known occurrences of this phenon are from silty or muddy sediments, whereas almost all other fossil samples of C. vesiculosus came from coarser sediments such as medium- or coarse-grained sands and gravels. At the localities of Kg 1 and Kg 2, the present phenon occurs together with abundant individuals of Glycymeris rotunda (Dunker), Venus (Ventricoloidea) foveolata (Sowerby) and Clementia papyracea (Gray).

Explanation of Plate 7
(All figures × 1.5, unless otherwise stated)
Figs. 1-10. Cryptopecten vesiculosus vesiculosus (Dunker)
Fig. 1. Right valve (UMUT CM16057a), Phenotype Q (rough subphenotype), sample Ms, Holocene Moeshima Shell Bed at Moeshima Islet near Kagoshima.
Fig. 2. Left valve (UMUT CM16057b), Phenotype Q (rough subphenotype), the same sample.
Fig. 3, Right valve (UMUT CM16057c), Phenotype Q (smooth subphenotype), the same sample.
Fig. 4. Left valve (UMUT CM16057d), Phenotype Q (smooth subphenotype), the same sample.
Fig. 5. Right valve (UMUT CM16057e), Phenotype R, the same sample.
Fig. 6. Left valve (UMUT CM16057f), Phenotype R, the same sample.
Fig. 7. Right valve (UMUT CM16038a), Phenotype Q,, sample Tm 2, Middle Pleistocene Umegase Formation at Tsujimori of Boso Peninsula.
Fig. 8. Left valve (UMUT CM16038b), Phenotype Q,, the same sample.
Fig. 9. Conjoined valves (UMUT CM16030a), Phenotype Q., sample Ik, Late Pliocene Shinzato Formation at Ikei Islet, Okinawa, 9a: external view of right valve, 9b: external view of left valve, 9c: anterior view, 9d: dorsal view.
Fig. 10. Conjoined valves (UMUT CM16030b), Phenotype Q,, the same sample; external view of right valve.
Figs. 11,12. Cryptopecten vesiculosus makiyamaisubsp. nov.
Fig. 11. Right valve (UMUT CM16033b), Paratype, sample Kg 1, Late Pliocene Hosoya Silt of Kakegawa Group at west of Ugari near Fukuroi.(×1)
Fig. 12. Left valve (UMUT CM16033a), Holotype, the same sample ( × 1) |


As the result of a number of dredge trials in the coastal area of Sagami Bay, assemblages of molluscs were investigated by Horikoshi (1957, 1960), Biological Laboratory of the Imperial Household (1971) and myself, and it was found that G. rotunda and V. (V.) foveolata live together at many muddy bottom stations, but that C. vesiculosus is restricted to sandy substrate and is only rarely accompanied by the other two species. At the type locality of the Jizôdô Formation, C. vesiculosus and G. rotunda occur in adjacent but clearly different fossil beds; the pectinid is abundantly contained in the coarse-grained sandy bed with another glycymeridid, Glycymeris (Tucetilla) pilsbryi (Yokoyama), but never found in the superjacent silty bed where G. rotunda is very common. Therefore, the samples Kg 1 and Kg 2 are unique not only in terms of morphological characters but also with respect to inferable ecologic habit. At present I regard these two samples as representing populations differentiated from the main stock of C. vesiculosus through adaptation to relatively fine-grained substrates. They are provisionally treated here as a new subspecies, C. vesiculosus makiyamai, though it is not impossible that this phenon is an incipient species, or merely a product of ecological variation.
The other aberrant fossil form is represented by the sample Kk (S) from the Wan Formation of the Ryukyu Group at Kamikatetsu of Kikai Island. This sample is morphologically distinguishable from any sample of C, vesiculosus in the much weaker convexity of the two valves, fewer radial ribs, more prominent and granular imbricated scales on the ribs, and several other characters (Plate 8, Figs. 1-4). This phenon occurs dominantly in coral sand in association with C. nux and numerous other molluscs (Nomura and Zinbo, 1934), larger foraminifers, calcareous algae, etc.

Explanation of Plate 8
(All figures × 1.5, unless otherwise stated)
Figs. 1-5. Cryptopecten spinosus sp. nov.
Fig. 1. Right valve (UMUT CM16170d), Paratype, sample Kk (S), Late Pleistocene Wan Formation at Kamikatetsu, Kikai Island; 1a: external view, 1b: internal view.
Fig. 2. Left valve (UMUT CM16170c), Paratype, the same sample; 2a: external view, 2b: internal view, 2c: dorsal view, 2d: anterior view.
Fig. 3. Right valve (UMUT CM16170e), Holotype, the same sample; 3a: external view, 3b: internal view, 3c: dorsal view, 3d: anterior view, 3e: byssal wing. ( × 4)
Fig. 4. Left valve (UMUT CM16170f), Paratype, the same sample; 4a: external view, 4b: internal view.
Fig, 5. Ventral ar ea of right valve (UMUT CM16170g), Paratype, the same sample. ( × 4)
Fig. 6-9. Cryptopecten yanagawaensis (Nomura and Zinbo)
Fig. 6. Right valve (UMUT CM16171a), sample Mn (Y), Middle Miocene Moniwa Formation at Junishin near Natori.
Fig. 7. Left valve (UMUT CM16171b), the same sample.
Fig. 8. Left valve (UMUT CM16171c), the same sample.
Fig. 9. Right valve (UMUT CM16171d), the same sample. |


In addition to these marked differences in the morphology and inferred ecologic habit, this form is regarded as specifically distinct from C. vesiculosus for the following reason. According to Sakanoue et al. (1967) and Omura (1983), and the age of this fossil bed is Late Pleistocene (about 0.08 Ma). All the Late Pleistocene and later samples of C. vesiculosus in various areas of Japan are, as noted before, composed of the two discrete phenotypes Q and R, while the sample Kk (S) is decidedly monomorphic and consists entirely of the individuals comparable with the Phenotype Q in spite of its young age and large sample size. Consequently, the reproductive isolation of this phenon from C. vesiculosus was probably completed before the spread of the phenotype R. In this article I describe this peculiar form as C. spinosus sp. nov.
Judging from its absence in Recent seas, C. spinosus was probably a dead-end branch derived from the main stock of C. vesiculosus and was unable to replace this parental species. Because C. spinosus is now represented by a large but solitary fossil sample, it is not known whether this was actually a species endemic to the Ryukyu Islands or whether the present sample represents only an immigrant population of a species widely distributed in tropical regions. Further research of Quaternary faunas in southeastern Asia may be necessary in order to solve this problem.
The Late Pleistocene sample Sm (N) seems to represent an aberrant phenon of C. nux stock, and is characterized by much stronger convexity of the two valves and less de
veloped spiny scales on the byssal wing than is found in other fossil and Recent samples of C. nux. This sample is here referred to as C. nux sematensis. C. nux nux does not occur at the type locality of this subspecies. Since the center of distribution of C. nux is believed to be in the equatorial Pacific rather than the Japanese waters, this subspecies probably represents a peripheral population near the northern limit of distribution.
The specimens of C. nux from the Red Sea (USNM 764165) seem to be characterized, on the contrary, by unusually tall, weakly inflated and equiconvex shell in comparison with ordinary samples from the Indo-Pacific. Though a subspecific name is not proposed here, the Red Sea population may also represent a peripheral isolate which is now undergoing speciation. It is also interesting to see that the samples of this species from Queensland, also a peripheral area of distribution, show somewhat specialized morphology (see Plate 9, Figs. 2, 5).

In conclusion, the present data of Cryptopecten are still too insufficient to test the model of allopatric speciation. It may be said, however, that various facts about the morphology and distribution can be explained to a considerable extent by this theory. At the same time, it is supposed that the short-ranging taxa newly recognized in this study represent only a minor fraction of the speciation and peripheral isolation that have actually occurred.