CHAPTER 6
Reproductive Season and Growth Rings of Cryptopecten
|
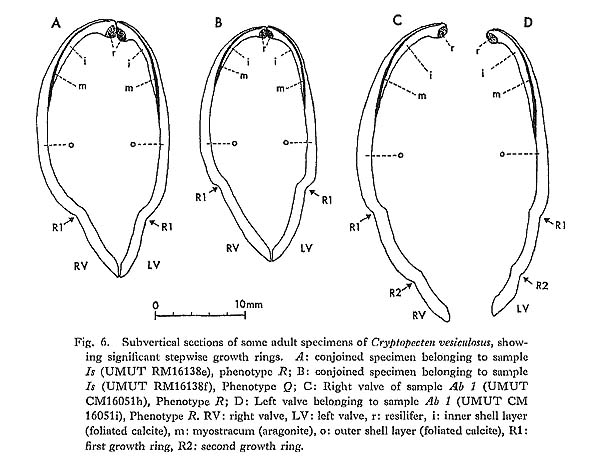
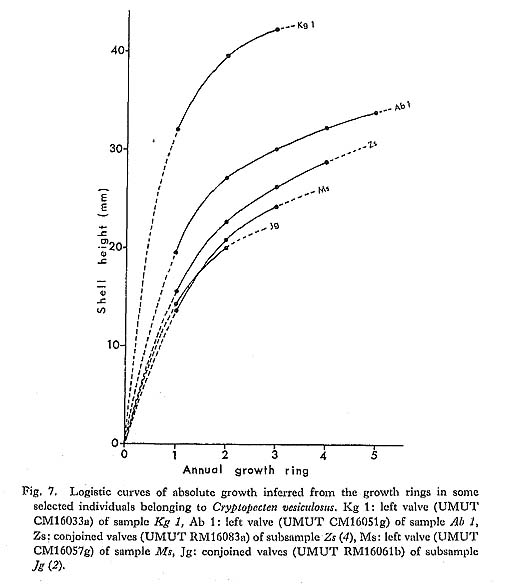
Various kind of basic information about the reproductive strategy and life cycle in living populations is of prime importance in order to understand the evolution of fossil organisms. Since Cryptopecten is mainly composed of lower sublittoral and bathyal species, direct embryological observation is difficult. My knowledge on this matter is still restricted to the anatomical features and their relation to shell morphology in some dredged samples of C. vesiculosus and C. bullatus. As shown in Table 1, numerous living individuals of C. vesiculosus, belonging to 26 subsamples, were collected from a sandy bottom-almost all at the same station-about 2.5 km west of the western end of Jôgashima Islet in the eastern part of Sagami Bay [sample Jg, 35°07.9'N, 139°35.1'E, 80-90 m]. They were obtained in the following six different seasons: Though the greater part of them are immature and probably yearling, all the large indi-viduals collected in summer possess well developed gonad. Therefore, this station can definitely be regarded as being situated in an area where reproduction actually occurs. The Pectinidae are sometimes said to have sexes represented in separate individuals (Drew, 1906) but to be more commonly hermaphroditic. Hermaphroditic reproductive glands are known in Pecten (Pecten) maximus and Aequipecten opercularis from England (Dakins, 1909; Rees in Cox, 1957; etc.) and also in two commercially important Japanese species, Pecten (Notovola) albicans and Patinopecten (Mizuhopecten) yessoensis. They have also been found in C. vesiculosus and C. bullatus. There are few characteristics worthy of special notice with regard to the anatomical features of C. vesiculosus and C. bullatus; they are fundamentally similar to those of ordinary species of Chlamys and Aequipecten. Tentacles, though not numerous, are considerably long. Eyes arranged at irregular intervals along the edge of the mantle are proportionally large, presumably reflecting deep (dark) habitat. Of the above-mentioned living materials of C. vesiculosus, all the relatively large-sized individuals (over 13 mm in length or height) in the July and August subsamples, irrespec-tive of phenotype and the presence or not of growth ring(s) on the surface, have a large swollen crescentic gonad attached to the adductor muscle. Its proximal part is creamy and certainly represents the male reproductive gland (testis), whereas its distal part is orange-vermilion and contains the female reproductive gland (ovary) (see Frontispiece). The boundary between the two glands is irregular but generally sharp. Both reproductive glands are also developed in some large individuals of the May subsamples, but not in any of those of the March, October and November ones. The spawning season must therefore be mainly in summer (or early autumn). It is likely that, as commonly seen in other hermaphroditic bivalves, the male gland becomes mature prior to the female gland within the same individual, so that self-fertilization is avoided. Most Pleistocene, Holocene and Recent large individuals of C. vesiculosus from Japan (over 20 mm in length and height) have one or more strong growth rings on the surface. The rings are often so conspicuous that the shells appear to be constricted, as seen in the profiles (Plates 5 and 6) and cross sections (Fig. 6). The curvature of shell surface, both external and internal, commonly changes drastically at the ring; it is strongest just before the formation of each ring and becomes suddenly much weaker after the formation. Judging from the periodicity of growth increments in the outer shell layer, it is inferred that rings are laid at times of unfavorable growth conditions. In many fossil and Recent large individuals several (five at the maximum) rings are clearly observed, and the inter-vals become regularly narrower with growth. The distance from the origin of growth to each ring seems to agree well with a logistic curve (several examples are shown in Fig. 7), which may be a general pattern of absolute growth (Simpson, Roe and Lewontin, 1960, fig. 49). Though the growth pattern before the formation of the first ring (or sigmoidal part of a logistic curve) is actually unknown, periodical formation of growth rings is also strongly suggested from the biometrical ground.
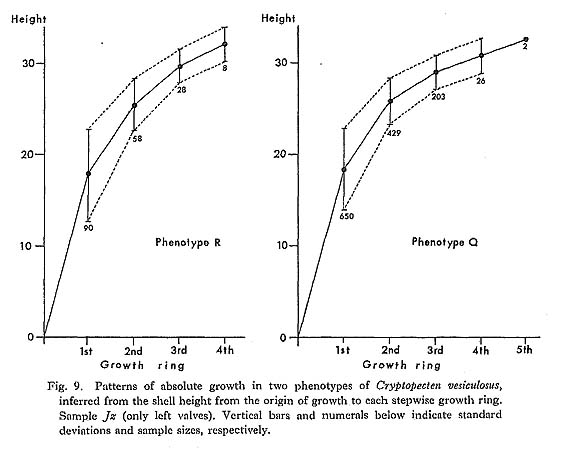
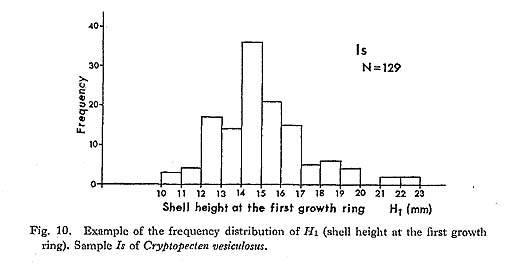
At first I presumed that these rings were formed in winter, simply because C. vesicu-losus is a warm-current species. Contrary to my expectation however, as a result of my examination on living populations in Sagami Bay, it was found that a growth ring is formed between early August and early October. More precisely, summer specimens with fully developed reproductive grands always show strongly inflated ventral peri-phery. It is therefore reasonable to consider that the increased internal space produced by the unusually strong curvature corresponds to the full development of the gonad. I presume further that in this season shell growth ceases or is much retarded in relation to minimized metabolism. As seen in most large individuals of the October subsamples, the quantity of soft part greatly decreases in autumn. In October very small individuals, which probably represent a new generation, are found together with large individuals exhibiting slight shell growth begun after the completion of the last ring. The present observation about the formation of growth rings seems to be consistent with the morphological features in several other dated living samples [Kz, Ma, Aj (1, 2), Os, Zs (1, 3, 4, 8), Hs (2), Ts (2, 3), Hk, Mb], which were collected at several stations along the Pacific coast of central Honshu in various seasons. Among these are three small samples were obtained in summer from Zenisu, a deep-water bank (ca. 100 m) southwest of KÔzu Island. Their soft parts being completely dried, I was unable to observe the de-velopment of reproductive glands, but it is noticed that the individuals of Zs (3, 4) collected on July 10, 1969 show strong curvature of shell near the ventral margin indicating the stage during or just before the formation of a growth ring. On the other hand, the individuals of Zs (8) collected on August 3, 1971 have a bit of added shell after the last ring. In an individual of Hs (2), which was collected on July 12, 1967 on another bank near Zenisu, however, slight shell growth already began after the formation of the last ring. Judging from the relation between the collecting dates and the growth amount after the last ring, spawning is generally considered to occur mainly in summer in sea areas other than Sagami Bay as well, but the season may vary slightly with the area, year and individual. It is quite likely that the reproductive season is somewhat earlier in southern populations, provided that spawning occurs under impetus from an increase in water temperature. Incidentally, the breeding seasons of such commercially important pectinids as Pecten (Notovola) albicans, Patinopecten (Mizuhopecten) yessoensis and Chlamys faneri nipponensis are considerably different in the various sea areas around Japan (Yoshida, 1964; etc.). The present data are still insufficient to permit reconstruction of life cycle and popula-tion structure. Since 10 mm mesh nets were used for dredging, size distribution of smaller individuals is difficult to determine. In July and August subsamples well-developed gonads are observed not only in the individuals with growth ring(s) but also in a considerable number of medium-sized individuals without a ring. These individuals are, however, accompanied by still smaller ones without any developed gonad or any inflation of ventral periphery. Therefore it can be most likely, even though not conclusive, that most individuals reach sexual maturity in the second summer. The distance from the hinge-line to the first growth ring (H1) varies greatly within each sample. This may be due to a long-term breeding season, but further investigation by regular sampling may be needed, since, as discussed later, the frequency distribution of H1 is sometimes clearly bimodal. The seasonality of spawning as well as the formation of growth rings has been inten-sively studied in Pecten (Pecten) maximus in the Irish Sea. According to Mason (1957), who examined regular samples collected at weekly or fortnightly intervals over two years, the scallop grows from spring to December and ceases growing in winter. Growth rings are laid down annually in early spring when water temperature is lowest. The spawning season of this species in this sea area is quite long, being mostly in late August or Sep-tember, but there is also less common spawning in the period between April and early August. Mason's observation seems, therefore, to indicate that the formation of growth rings is unrelated to the breeding season, an indication, however, which is somewhat in contradiction to Ree's (1957) interpretation. Mason (1957) cited his 1953 thesis: "Spawning cannot possibly be responsible for the cessations of growth in the animal's first two winters, since scallops do not spawn for the first time until the summer after the deposition of the second growth-rings." Because C. vesiculosus has much smaller shell size and much fewer growth rings than P. (P.) maximus, it is only natural to assume that sexual maturity is attained somewhat earlier. Growth rings which I have observed in several specimens of P. (P.) maximus pre-served in our museum and several other institutions, are not accompanied by periodical change of surface convexity. This is also the case with many other pectinids. Such rings, I presume, are not related to breeding. However, remarkably stepwise growth rings, strikingly similar to those of C. vesiculosus, are frequently met with in Chlamys (Swifto-pecten) swiftii (Bernardi, 1858) and Nodipecten nodosus (Linnaeus, 1758). Among Japa-nese fossil species such remarkable growth rings appear in Chlamys cosibensis (Yokoyama, 1911) and its relatives from the Miocene-Pleistocene (see Masuda, 1959). The origin of stepwise growth rings should be more intensively studied in each species, but analogy with C. vesiculosus leads me to suspect that they are primarily related to the development of gonads and temporal cessation of growth after annual spawning. In bivalves growth rings undoubtedly arise from various causes. In C. vesiculosus, however, it is concluded that in adult individuals they are laid down annually, mainly in summer or early autumn, only during the season of reproductive activity and also that their number indicates the lowest possible age of each individual. This conclusion may be applicable to fossil samples of this species, e. g. in comparison of age composition between samples and the evaluation of seasonal heterogeneity. The relative abundance of small individuals without any growth ring in the sample Jg (1-26) seems to indicate a high mortality rate in the immature stage. Since the sample was obtained using nets of 10 mm mesh, there is doubtless a greater abundance of im-mature individuals than the number collected would indicate. Of 265 living individuals in this sample only 22 have only one growth ring. Also noteworthy is the fact that many dead shells of medium size, between 12 mm and 20 mm, show strongly convex ventral periphery, suggesting that many mature individuals passed away just after the first spawning, only a fraction surviving to join in reproductive activity in the following summer. This may not necessarily be true however in other living and fossil populations. Large specimens with more than two rings are quite common in many Pleistocene fossil samples from Boso Peninsula (e. g. samples Sn 1-3, Ny 1-4, Jz, Ic and Ab 1-2) as well as in some Recent ones (e. g. Zs, Ts and Az). For example, as shown in Text-fig. 9, of 912 collected left valves of fossil sample Jz, 740, 487, 231, 34 and 2 individuals possess one, two, three, four and five growth rings, respectively. No significant difference exists between the growth patterns of the two phenotypes. Because of the sampling bias and post-mortem sorting, these figures do not seem to be directly indicative of mortality rate, but it is evident that the average number of growth rings is generally much larger in comparison with Recent population samples. The mortality rate and average span of one generation may vary considerably among local populations in time and space. In most Pliocene samples (e. g., Sh 1-2, Nj, Ik and Kg 1-2) growth rings, if present, are comparatively weak (Plate 7, Figs. 9-12). It is, however, doubtful whether the difference is entirely attributable to phyletic change, because weak rings are also common in some Recent samples from the southern East China Sea (e. g., Ec, My)
Also noteworthy is the fact that several specimens (about 13 mm in length and height) of C. bullatus, which were collected on June 15, 1983 off the coast of Shirahama of Kii Peninsula (sample Sr (2, 3) (B)), possess a considerably developed gonad (see Table 2 and Frontispiece). An associated specimen of C. vesiculosus (sample Sr) however, which was obtained by the same dredge as Sr (2) (B), does not show any developed gonad, though its shell size is considerably large. The reproductive seasons of the two species are probably different, at least in this sea area. Large specimens of C. bullatus, C. yanagawaensis and C. spinosus often have one or two stepwise growth rings, which are, however, less conspicuous than those of C. vesiculosus. In C. nux growth ring(s) are rarely encountered even in such large population samples as Kk (N) and Bh (N). Judging from the small ultimate size and occasionally strongly inflated ventral part, I presume that almost all the mature individuals of this species pass away immediately after the first spawning. |