Re-evaluation of specimens of plants
As a researcher of developmental genetics in a model plant, Arabidopsis thaliana (L.) Heynh., who also studies the biodiversity of plants, I wish to discuss how specimens can be used in modern reseach. In the era of von Siebold, dried specimens with descriptions on the label supplied sufficient information on the features of plants, such as locality, morphology, colour, dimensions, and so on. Natural variation in a particular taxon was represented by the variation presented in a number of dried specimens in herbaria. The standard manner of preparation of herbarium specimens suited the analytical interests of plant biologists, since only a few methodologies, namely morphology or geobotany, were used at the time to understand plant species.
To more deeply understand plants, we now use various research methods that did not exist in the classic era. Because classic herbarium specimens were not collected to be analyzed by modern techniques, many of the newer methods cannot be applied to classic herbarium specimens. For example, although useful information can be obtained from DNA sequences, as reviewed by Mishler (2000), DNA cannot be extracted from most older herbarium specimens. If separate DNA collections are made they can be quite useful and informative and will greatly enhance the value of a specimen. DNA samples complement the morphological and other information associated with specimens. In consideration of the importance of DNA data in modern plant taxonomy, in the first part of this article, I will discuss how DNA samples can be made in the field to supplement traditional herbarium specimen. DNA data alone is not sufficient and to represent the plant from which it was taken, a complete voucher specimen with detailed collection information should be made and deposited in a recognized herbarium where it is is accessible to researchers. Considering the importance of epigenetic mutations on morphological variation, a profile of the mRNA transcripts can also complement the information on herbarium specimens. Most herbaria are currently not equiped to deal with the additional kinds of material required for DNA studies and the curation of molecular materials, both in terms of facilities and in staffing, should be considered when new herbaria are designed. DNA samples should also be distributed to researchers worldwide. Distribution may be conducted by bioresource centers, as is done in the study of model species such as A. thaliana. In the second part of this article, I will discuss the establishment of a stock center for molecular phylogenetic studies of plants. Finally, I will discuss the kind of information that can be combined with herbarium specimens. These discussion will help to redefine the concept of herbarium specimens.
Case I: Molecular analysis of hybridization and introgression between Callicarpa japonica and C. mollis (Verbenaceae).
In June 2000, I encountered some individuals of Callicarpa (Fig. 1) on Mt. Kinugasa, Atsumi Peninsula, central Japan, that were morphologically intermediate between C.japonica and C. mollis. As shown in Figure 2, they showed great variation, suggesting introgression or repeated hybridization between the two species. To confirm my suspicions, I collected voucher specimen and DNA samples of 62 individuals. Dr. Jun Yokoyama of Tohoku University and I extracted the DNA, conducted PCR reactions and sequencenced the amplified DNA. Analysis of three nuclear ITS1, ADH, and chloroplast trnL-F DNA confirmed introgression between Callicarpa japonica and C. mollis on Mt. Kinugasa (Tsukaya et al., 2003). In addition, we also found unexpectedly that all C.mollis in this area shows genetic contamination from C.japonica. Since no morphological abnormalities could be seen in individuals of C.mollis in the area, it appears that the identification of C.mollis on the basis of morphology cannot be supported by molecular data. To find pure C.mollis, we analyzed many other individuals of C. mollis from other parts of Japan.

Fig. 1. A typical individual of Callicarpa that has intermediate features of C. japonica and C. mollis. Note large sepals and pubescent abaxial side of leaves and stems which show genetic influence from C. mollis, and large inflorescence which show genetic influence from C. japonica. Photograph was taken in Mt. Kinugasa, Atsumi Peninsula, central Japan, in November 9, 2003, by the author.
Here, I should point the problem of availability of DNA samples. We can analyze many species of plants from the viewpoint of morphology and geobotany by visiting large herbaria, but if we want to analyze plants using DNA sequences, we must collect the specimens and DNA by ourselves by visiting various localities unless the curators of herbaria permit us to remove DNA samples from specimens. Moreover, even when curators permit the removal of DNA material from specimens, old specimens are rarely useful for molecular analysis because of improper preparation at the time of collecting. This is one of the shortfalls of classic herbarium specimens that I mentioned at the beginning of this article. Those who conduct DNA analaysis from herbarium specimens should also consider the source of the material they remove. Some lab workers consider all herbarium specimens to be fair game from which samples can be harvested for DNA analysis, yet they themselves rarely, if ever, collect herbarium specimens for the collections they parasitize.
Fortunately, Callicarpa mollis is not rare and it is easily accessible in Japan. We were able to collect a sufficient amount of material and were able to find pure C.mollis from the point of view of molecular markers, confirming the introgression on Mt. Kinugasa and proving that hybridization and introgression between C.mollis and C. japonica are common in Japan (Tsukaya et al, 2003).

Fig. 2. A part of variation in morphology of inflorescence among individuals of Callicarpa found in Mt. Kinugasa, Atsumi Peninsula, central Japan. Upper left panel shows a typical C.japonica, while the others show characters of C. mollis more and less, by hairs on sepals and stems, and small number of flowers per inflorescence. Flowering time also varied. Bar, 1 cm. Phtotograph was taken in June 4, 2000, by the author.
Case II: Diversity in size of leaves in Mitchella undulata (Rubiaceae)
The second case is similar to the above and clearly shows the difficulty of obtaining molecular information from herbarium specimens. Mitchella undulata Siebold et Zucc. has been treated as two varieities, var. undulata and var. minor Masamune, the latter of which is a dwarf form on Yakushima, Kagoshima Pref., Kyushu, Japan (Fig. 3). During studies of dwarfism in plants from Yakushima, we reevaluated the treatment of M. undulata. Dried specimens in herbarium of the University of Tokyo (TI) were useful for determining size variation. We measured leaves at the nodes bearing flowers or fruits. We concluded that the dwarf form should not be recognized taxonomically. Although individuals on Yakushima are the smallest in size, variation is continuous among all individuals of the species (Fig. 4). Simultaneously, we carried out histology of the leaves in plants from several localities. We found that all variation in size in the leaves examined could be attributed to variation in the number of cells in the lamina (Tsukaya, 2002a, 2002b; Yokoyama et al., 2003). We speculate that the system(s) that controls cell proliferation in the leaf lamina varies among individuals in Japan (Yokoyama et al., 2003). We also obtained unexpected results from molecular analysis of the samples. It was speculated that there are at least two genetic groups in this species in Japan (Yokoyama et al., 2003; Fig. 5). The two genetic types cannot be distinguished by morphology and size variation is not linked to the genetic groups. To further understand the geological distribution of these two molecular types in Japan, we need more samples for DNA analysis. The study is still underway.

Fig. 3. Size variation in Mitchella undulata Siebpld et Zucc. Specimens were collected from Okazaki, Aichi Pref.; Mt. Kunimi, Mie Pref.; and Yakushima Island, Kagoshima Pref., in October 2002, from left to right. The smallest form represented in the specimen from Yakushima Island has been treated as M. undulata var. minor. Unit of scale is cm.
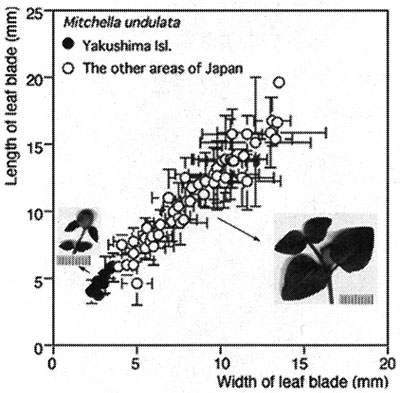
Fig. 4. Leaf size variation in Mitchella undulata Siebold et Zucc. from various localities in Japan. Each data point represents the mean of data from one locality. Unit of scales, 1 mm. Modified from Yokoyama et al. (2003).
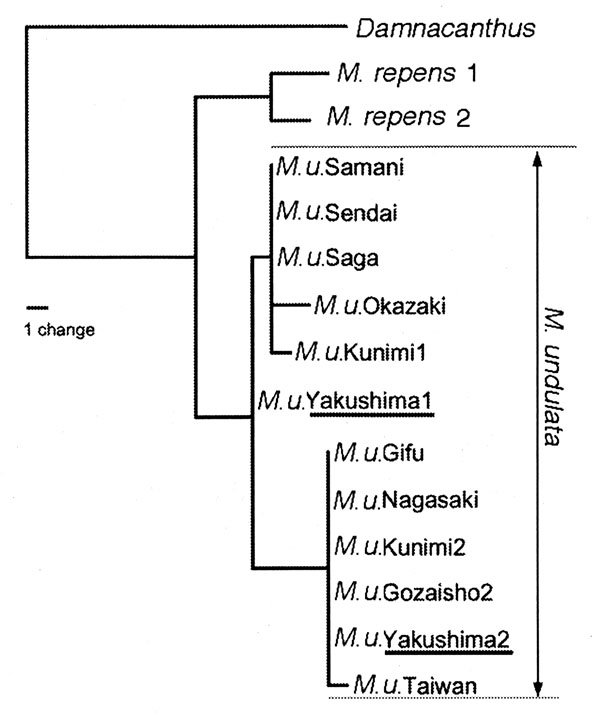
Fig. 5. One of the 1650 most parsimonious phylogenetic trees (MP method) based on combined data from ITS1 and ITS2 sequences of Mitchella undulata Siebold et Zucc. [47 steps, CI (excluding uninformative sites) = 0.93; RI = 0.95]. Twelve samples of M. undulata, shown as M. u. with name of its locality, were analyzed. M. repens and Damnacanthus indicus are the outgroups. Modified from Yokoyama et al. (2003).