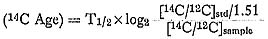CHAPTER 10
14C Dating by Accelerator Mass Spectrometry of Carbonized Plant Remains from a Middle Paleolithic Hearth at Douara Cave, Syria
Koichi Kobayashi, Kunio Yoshida, Hisao Nagai, Mineo Imamura, Hideki Yoshikawa, Hiroshi Yamashita, Shohei Ohizaki, Singo Yagi, Takayuki Kobayashi, and Masatake Honda
10. 1 INTRODUCTIONA number of radiocarbon dates, obtained by using a conventional β-counting method, have been reported concerning samples from Middle Paleolithic horizons in Douara Cave, Syria (Akazawa, 1979). Two dates on ostrich eggshells were 46,700 +2,200/- 1,700 BP and >53,800 BP (GrN-8638, GrN-8058 respectively). Two dates on hearth ash materials were scaled out at >43,200 BP (TK-166, -167, -168) and >52,000 BP (GrN-7599), but another (TK-165) gave the result, 38,900 ±1,700 BP. In addition, a date of 75,000 BP was obtained by a different method (fission track dating) on a barite pebble taken from the same hearth deposit as the ash samples noted above (Nishimura, 1979). In order to examine this discrepancy in the chronological results we have turned to a third method, accelerator mass spectrometry, and used samples of carbonized seeds, Many pieces of these, which have been identified as Celtis spp., were also collected from the same hearth deposit as the ash and barite pebble (see Chapter 9 in this volume). At present, there are no Celtis trees around Douara Cave or elsewhere in Palmyra Basin, but they can be found near the Mediterranean coast several hundred km away. The accelerator mass Spectrometry (AMS) technique which we used was recently developed using the tandem Van de Graaff accelerator in the Research Center for Nuclear Science and Technology at the University of Tokyo (Imamura, 1984). Since the expected age was so old that natural contamination of the sample by recent carbon, and contami nation by modern carbon in the process of sample preparation, were anticipated, chemical treatments to attempt to eliminate theae were necessary, and are described below. It should also be noted at the outset that the date we eventually obtained cannot be considered the final result, since we are trying several other sample preparation treatments, and we are also developing the AMS system in order to lower the background level which limits the maximum measurable age. 10. 2 ACCELERATOR MASS SPECTROMETRYCosmic-ray produced, long-lived, radioisotopes, such as 10Be, 14C, 26Al, 36Cl, 59Ni, 53Mn and 129I carry various pieces of information about age, origin, nature etc. of geological, astronomical and archaeological samples. As the activity of these isotopes in natural samples which are able to be handled is very weak it is usually measured by the use of low-back-ground decay counting systems. However, it is clear that to count the numbers of long-lived isotopes directly is much more efficient and sensitive than to measure the activity conventionally by waiting for isotope-decay (Muller, 1977). During the past decade techniques to detect directly the long-lived radioisotopes have been developed all over the world (Gove, 1978, Kutschera, 1981, Wölfli, 1984); those which use heavy ion accelerators are called AMS.
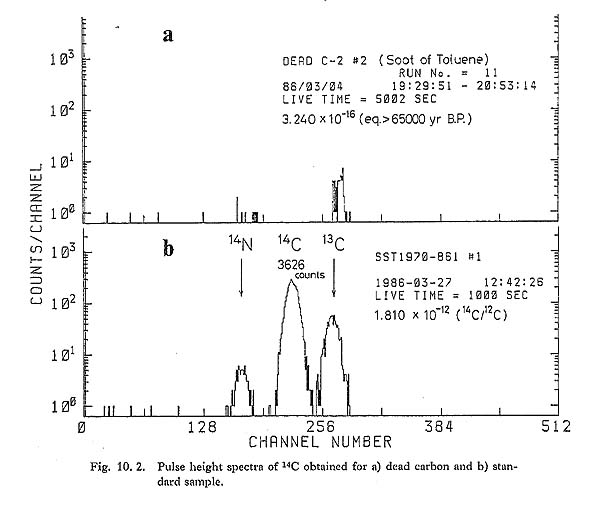
Detection of long-lived radioisotopes by AMS is so sensitive that it is possible to use much smaller samples (usually only 0.001 as large) and to measure much older ages, than in the conventional decay counting method. The principles of AMS and the reasons why the method is so sensitive have been described elsewhere in detail (Gove, 1978, Muller, 1979, Purser, 1979, Litherland, 1980). They can be summarized as follows. 1) Accelerating isotopes to high energy makes it possible to distinguish isobars such as 14C and 14N from each other by measuring their different ionization rates in matter. 2) Molecular ions of the same mass-to-charge ratio as the ions in question, such as 13CH and 12CH2 ions for 14C ions, can be fragmented into atomic ions during the process of acceleration. 3) Since the total energy of each accelerated ion can be measured, it is possible to obtain more information about the identification of the isotopes. In 1979 we started a project to make an AMS system using the 5MV tandem accelerator at the University of Tokyo. The initial aim of the project was to detect 10Be and 14C very precisely, and then to detect 26Al, and 36Cl etc. By 1982 our unique technique, called an internal beam monitor method, was successfully developed, and it provided sensitive and very precise measurement of 10Be/9Be (Imamura, 1984). In 1983 an earthquake caused the shutdown of the tandem accelerator, but our project resumed in 1985 and we developed the system for precise detection of 14C. The instrumental configuration and method for 14C measurement is almost the same as that used to measure 10Be concentration, and since the details of the latter system have been reported elsewhere (Imamura, 1984) only an outline is given below. To determine the age of a sample, a ratio of 14C/12C must be measured that is as small as 10-12 < 14C/12C < 10-16. In practice both 14C and 13C ions are accelerated and the currents are measured by a heavy ion counter and a Faraday cup respectively. The ratio of 14C/12C can then be derived from the ratio of 14C/13C. The experimental layout of the system used for the 14C/12C measurements is illustrated schematically in Fig. 10. 1. Very small amounts of amorphous carbon (less than 1 mg), extracted from the samples, arc pressed into sample holders in the ion source (Cs sputtering negative ion source) and ionized to negative ions of C-, CH- etc. Ions of m/e= 14 that are 14C- and 13CH- etc. are separated from other ions by a 90° magnet and injected into the tandem accelerator. The terminal voltage of the accelerator is set to 3 MV. After acceleration to this voltage, the high charge states of the ions are converted from negative to positive in an Ar gas stripper canal, which is located in the high voltage terminal, and the 13CH- molecular ions are fragmented into atomic ions with positive high charges. Next, following acceleration in the positive ion stage, the 14C3+ and 13C3+ beams are selected by the 90 ° beam analyzing magnet. In the beam monitor system the 13C3+ beam is measured by a Faraday cup, the position of which can be adjusted, and the monitor slit current is used to stabilize the terminal voltage while the main beam of very weak 14C3+ is allowed to pass through another set of slits in the main beam course. Fluctuation of the ion source current, and transmission from the ion source to the Faraday cup, is monitored by the 13C3+ current. The 14C3+ beam is then filtered through an electrostatic deflector to remove impure ions which were produced by charge-exchange reactions in the accelerating tubes. Finally, the beam passed through the deflector is focussed around a gas absorber which is directly in front of a heavy ion detector (silicon surface barrier detector). Making use of differences of energy loss for various ion species (the energy of the isobar ions which pass through the absorber is changed differentially), it becomes possible to distinguish the isobars from each other by measuring the residual energy of the ions with the heavy ion detector. In the case of 14C detection only a Havar foil window of 1.8 mg/cm2 thickness is enough to separate the energy of the isobar ions of 14N from that of 14C. Figure 10. 2: b shows a typical energy spectrum of the heavy ion detector obtained for a standard sample (SST-1970: the ratio of 14C/2C is equal to 1.51 times that of modern carbon. Provided by Prof. K. Kigoshi, Gakushuin Univ.). The 14C peak is clearly separated from the other peaks which are of 14N and 13C ions. The time required for measurement depends on the age of the sample, but in order to get good counting statistics it takes 1,000-7,000 seconds for one run.
Using the 14C ion counts obtained by the heavy ion counter and the 13C ion currents measured by the monitor Faraday cup, we can get 14C/13C values in the samples as follows:
where e is transmission efficiency between the beam monitor and the detector. Subscript m, d and s indicate the beam monitor, the detector and the ion source. RH is the ratio of CH- to C- which varies from sample to sample. I13/I12 is the ratio of the negative ion currents of m/e=13 and 12 respectively, which is measured at the ion source before and after injection into the accelerator. (13C)/(12C) is an isotopic abundance of natural carbon equal to 1.12×10-2.
Usually we repeat the procedure (run) a few times, and measure the standard sample before or after the run. By comparing the data with that from the standard samples we can calculate the age of the samples as follows:
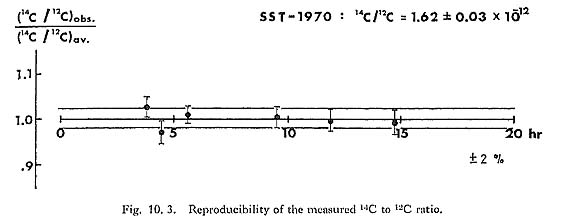
Figure 10. 2: a is an energy spectrum obtained for dead carbon (counting time: 5,000 seconds). As is shown in the Figure we did not get any 14C counts in this run. The "14C" signals which are counted in a dead carbon run constitute the so-called "background" which determines the maximum age we can reach. The background levels are not constant and fluctuate such that the maximum age varies from 55,000 to 67,000 years. It is very difficult to eliminate the 14C background levels completely because a trace of CO2 and organic gas exists everywhere, even in vacuum systems, and, what is more serious, the samples can easily be contaminated by modern carbon. The reproducibility error-range attained so far by using our method is less than ±2%. This is shown in Fig. 10. 3, which was compiled from data obtained by repeated measurement of the standard samples. 10. 3 SAMPLE PREPARATIONArchaeological samples for dating may be contaminated by 14C in several stages. In the site there will have been absorption of CO2 gas and carbonate, and of organic materials, and similar contamination occurs during preparation for AMS. It seems easy to remove CO2 and carbonate by adding diluted HCl solution and, in any case, it is difficult to produce C- and CH- ions from CaCO3. Organic contaminants are more complicated. Alkali and acid soluble components are easily eliminated, but cellulose tissue, for instance, cannot be removed by this treatment, and the problem is accentuated by the small size of samples for AMS.
For our purposes, all chemical processing of samples is done in a box made of lucite. The glass-ware we use is made of fused quartz and other laboratory-wares are made of Teflon or polyethylene. In the Douara Cave samples we analyzed there are apparently two types of material. LITERATURE CITED
|