APPENDIX I GAKUSHUIN UNIVERSITY RADIOCARBON DATINGS
Kunihiko KIGOSHI
Department of Chemistry, Faculty of Science, Gakushuin University
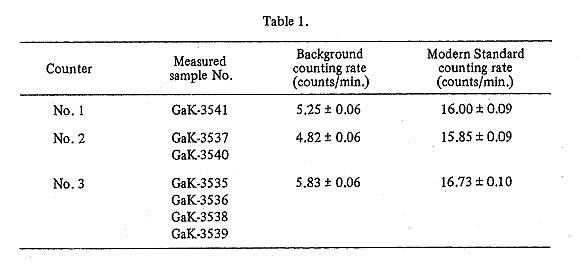
Radiocarbon measurements and calculation of agesThe beta radioactivities of carbon-14 in the samples were measured by the acetylene filled proportional counters. The proportional counters used for the datings were three counters (Nos. 1, 2, and 3). These counters were made by copper tubings of 50 mm diameter and 60 cm long, and shielded by multi-anode counters and iron shieldings of 25 cm thick. Th'è acetylene gas was filled in the range of 600 to 1000 mmHg, according to the amount of available carbon from the sample. The counting rates for dead carbon (background counting rate) and for modern standard sample (95% of NBS Standard Oxalic Acid) at 1000 mmHg are shown in Table 1 together with the sample numbers measured by each counter.
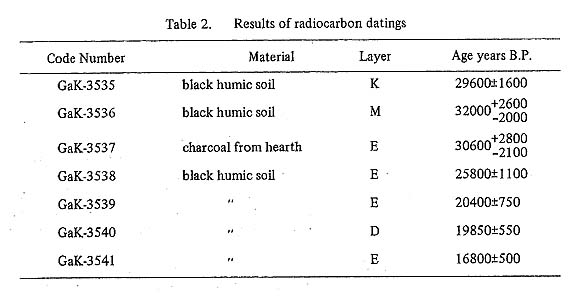
The calculation of ages is based on the Libby's half life of C-14,5570 years, and indicated ± errors are the years corresponding to the standard deviations (one sigma) of beta rays counting statistical errors. Chemical treatment on the samplesAll samples were at first boiled with 2N hydrochloric acid for 5 to 10 minutes in order to remove the inorganic carbonate. After washing with distilled water, samples were dried at 110°C. The dried soil sample was heated in oxygen stream to prepare carbon dioxide. The carbon dioxide was purified by calcium carbonate precipitation from the ammonium carbonate solution and decomposition of calcium carbonate by hydrochloric acid. Finally, carbon was converted to strontium carbonate and reduced by heating with magnesium powder in vacuum to strontium carbide. The acetylene was prepared by the reaction on the strontium carbide with tritium free water. The obtained dates are shown in Table 2.
comment on the measured datesThe stratigraphic order and measured dates are well ordered except GaK-3541 when we assume the short time interval between layer E and layer K. The soil sample of GaK-3541 was exceptionally low in its carbon content and its age should be most strongly affected by the contamination of younger materials from the upper levels. Also the other samples might be contaminated by the younger materials, but the effect of the contamination on age will be reduced by the large amount of carbon in the soil samples. When the younger organic material from the upper levels was absorbed in the layer in question, the evidence will be given by the difference of the ages obtained from the different materials, e.g. humic acid and humin, separated from the same soil sample. We did not check this difference on the soil samples from Douara Cave. Then, now we can only say that the measured age indicates a possible youngest age of the soil, namely true age might be older than the measured age. On this view point, there seems no essential contradiction between the dates of TK and those of GaK. |