APPENDIX II THE RADIOCARBON DATINGS OF TK-111(a), TK-111(b), and TK-112 BY THE UNIVERSITY OF TOKYO
Hiromi KOBAYASHI
Carbon Dating Laboratory, Department of Anthropology, Faculty of Science, The University of Tokyo
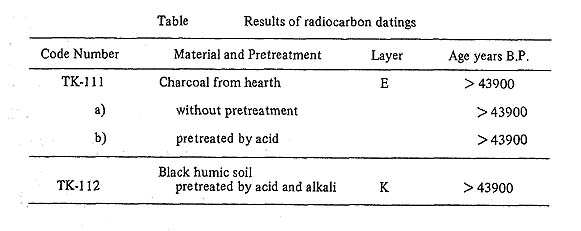
Radiocarbon measurement and its measurable limitThe radioactivities of the C14 were counted using a Houtermans-Oeshger type multi-anode anticoincidence proportional gas counter. The central counter tube is made of aluminized polyethylene foil (0.06 mm thick), with a 72 mm inside diameter and 300 mm sensitive length. It has several small holes (5 mm diameter). The external counter tube is made of stainless steel (4 mm thick), with 93 mm inside diameter and 350 mm length. The anode wires of both counters are made of stainless-steel (0.05 mm diameter). These counters are encased in a 250 mm shield of steel on all sides. Acetylene was used as the counting gas and always filled in the counter to 75.33 mm Hg at a temperature of 22 ±1°C The counting rates of the background and the 95% NBS oxalic acid standard were 1.25 ± 0,02 cpm and 14.39 ±0.12 cpm respectively. Ages were calculated using the C14 half-life of 5570 years. The statistical errors, corresponding to one standard deviation, include the contributions of the background, the NBS oxalic acid standard, and the sample count. An infinite date is given with a limit of 43,900 years B.P corresponding to the activity of 3 sigma, when the observed activity is less than 3 sigma above the background count. Chemical treatment of the samplesSample TK-111 was charcoal from hearth according to the sample submitter, but it had the appearance of black humic soil and seemed not to be so great in quantity. A 200 g portion of the sample without any pretreatment was first converted to CO2 by burning in a stream of oxygen. The following steps were then involved:
CO2+2NH4OH =(NH4)2CO3+H2O, Contrary to our expectation, sample TK-111 had high carbon content, so the rest of this sample was subjected to a pretreatment in which the sample was treated with cold 2N-HCl by the simple process of putting it into a beaker and pouring the acid over it to removed inorganic carbon contaminants. After termination of effervescence the HCl was taken off, the sample was rinsed thoroughly with distilled water, and dried in an oven at 130°C or less. The sample was then subjected to burning in a stream of oxygen. TK-111(a) was the sample without any pretreatment and TK-111(b) was the sample with the pretreatment. Sample TK-112 was black humic soil according to the sample submitter, but it contained something like small massive pieces of carbon powder which were first picked up, boiled with distilled water, treated with 0.2 % KOH, boiled with distilled water, treated with 0.1 N-HCl, boiled with distilled water and dried in an oven at 130°C or less. Subsequent chemical treatment was the same as for samples TK-111(a) and TK-111(b). Results are shown in the Table.
|