II. Material and Methods
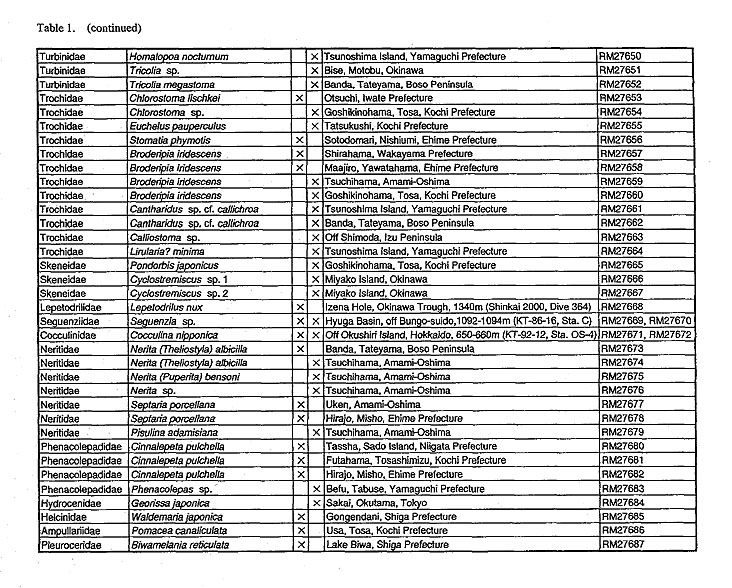
(1) MaterialA total of 52 species belonging to 43 genera of 19 families were selected as material to include diverse groups of Archaeogastropoda (Table 1). Observations were made on anatomical characters of 23 species (23 genera and 17 families) and protoconchs of 38 species (33 genera and 15 families).
Most specimens were collected in the intertidal or subtidal zone except some deep-sea and terrestrial taxa. Living specimens were relaxed in solution of magnesium chloride of various concentration to prevent the head-foot from retracting into the shell. Then animals were fixed in 10% formalin for several days or weeks. In the case of spiral forms, shells were broken with a hammer before fixation or dissolved in hydrochloric acid after fixation to enhance the effect of the fixative. Fixed animals were finally preserved in 70% ethanol. Protoconch samples were picked up from various sediments (shell sands) which were collected from intertidal or subtidal zones or from deeper habitats by dredging. Specimens used in this study were registered and preserved in the University Museum, University of Tokyo. (2) Morphological ObservationsSoft parts: The anatomy was reconstructed by a combination of the following three methods, (i) Animals were dissected under a binocular microscope. In fresh animals, some organs could be distinguished by color. Morphology of tentacles and lappets were also observed in anesthetized living animals. In the case of preserved specimens, dissected animals were observed by staining with methylene blue. (ii) Microanatomical details that were not observable by optical microscopy were investigated by scanning electron microscope (Hitachi S-2400, at 12-20 kV acceleration voltage). Dissected parts were dehydrated in a graded series of ethanol and t-butyl alcohol (2-methyl-2-propanol), and dried using a freeze dryer (Hitachi ES2030). Pieces were coated with platinum vanadium using an ion coater (Eiko IB-5). Some minute specimens were embedded in paraffin as for usual histological sectioning and cut at 0.1-0.5 mm with a steel knife. The soft parts were deembedded with xylene and freeze-dried for SEM observation, (iii) Serial sections were prepared to supplement gross dissection in Scutus, Sulculus, Chlorostoma, Nerita, Cinnalepeta, and Lepetodrilus. Paraffin-embedded serial sections were made at intervals of 6-10 µm and stained with haematoxylin and eosin. Radula: Radulae were removed from the buccal mass by soaking in sodium hypochlorite for several minutes and air-drying, following slight dehydration in 70% ethanol. The radular ribbon was cut into several pieces to reveal the basal morphology and relationships of overlapping teeth. SEM observation was carried out at 5-6 kV acceleration voltage. Protoconch: Morphological features of protoconchs were observed with SEM, with special reference to shape, number of whorls, sculpture, and boundary with the teleoconch. Morphology of the early teleoconch was also described by contrasting it to that of the protoconch. SEM observations were made at 12-15 kV acceleration voltage. (3) DescriptionOrgan systems: Arrangement and division of some characters are somewhat modified from conventional usage for convenience. (i) Various features on the exterior surface of the body are treated collectively as "external characters." Similarly, organs in the pallial cavity are allocated to the "pallial complex." Therefore, tentacles, lappets and other external sense organs are described as external characters, whereas the osphradium is assigned to the pallial complex, (ii) Buccal muscles are treated within the digestive system rather than the muscular system. This treatment stresses the unity of buccal-mass characters as a functional unit of the feeding apparatus. Terminology: Terminology and abbreviations adopted in this study are listed in Table 2. Terms are derived from anatomical texts on malacology for the most part (e.g. Fretter and Graham, 1962; 1994; Voltzow 1994), but some additgional notes are necessary. (i) Nomenclature onfbuccal musculature follows functional naming that divides muscles into protractor, retractor, levator, depressor, sphincter, dilator, and constrictor (e.g. Graham, 1964,1973; Fretter, 1965; Nisbet, 1973), rather than naming based on sites of insertion (e.g. Lemche and Wingstrand, 1959; Wingstrand, 1985). (ii) For the excretory organ, the term "kidney" is used in place of "nephridium" because of popularity (e.g. Pretter and Graham, 1962; 1994), although it is inappropriate zoologically (Haszprunar, 1988 b: 383). (iii) Terms for some characters specific to Neritopsina (e. g. annex and basal glands in the reproductive organ) follow Berry ef al. (1973) and Thompson (1980). (iv) Descriptive terms of orientation follow Ponder and Lindberg (1997) to compare non-gastropod "pretorsional" and gastropod "post-torsional" states. This distinction is particularly important in discussion considering the evolution of characters relative to anterior-posterior or right-left axes.
(4) Claditstic AnalysisOutgroups: Nautilus (Cephalopoda), Neopilina (Monoplacophora : Tryblidiida) and a generalized polyplacophoran were selected as outgroups of Archaeogastropoda, following Ponder and Lindberg (1996; 1997). Anatomical information of outgroups was mainly derived from Griffin (1900) for Nautilus, and Lemche and Wingstrand (1959) and Wingstrand (1985) for Neopilina (or Vema for several characters). The generalized characters of Polyplacophora were coded (under the name "Chiton") from various sources, mainly Hyman (1967), Salvini-Plawen (1985). and Eemisse and Reynolds (1994). Within Conchifera, Diasoma (Bivalvia and Scaphopoda) were eliminated from outgroups, because of entire loss of the buccal mass in the former and lack of data on buccal structures in the latter. In Gastropoda, two caenogastropod genera (Pomacea and Biwamelania) were added to test the relationship between Archaeogastropoda and the root of Caenogastropoda. Their states were determined by personal observations on Pomacea canaliculata (Lamarck, 1819) and Biwamelania reticulata Kajiyama and Habe, 1961 and partly by data from Berthold (1991). In the search for trees, Polyplacophora was designated as outgroup and others were included in the ingroup. Ingroup: The OTUs used in the cladistic analysis include 23 archaeogastropod genera selected to represent various families or superfamilies extensively. All patellogastropod families were anatomically investigated except Neolepetopsidae, Among the ten known families of two superfamilies of "Cocculiniformia," only Cocculinidae are represented (by Cocculina) in this study; other families were not treated because material was unavailable. Among vetigastropod groups, Crypeosectidae and Skeneidae were not used in the analysis, but all taxa at superfamilial level were considered. Seguenzioidea was not investigated in detail due to limited quality and quantity of material. States of several characters were supplemented by previously published data in literature (Quinn, 1983; Ponder and Lindberg, 1997). Among deep-sea limpet taxa, data for Neomphalus fretterae McLean, 1981 were constructed from the descriptions by Pretter et al. (1981) and McLean (1981). Peltospiroidea and Melanodrymia were not used, because character states have not been fully elucidated for characters required by this analysis. Lepetodriloidea were represented by Lepetodrilus nux (Okutani, Fujikura and Sasaki, 1993). In the neritopsine groups, only Neritoidea (Neritidae and Phenacolepadidae) and Helicinoidea (Helicinidae) were used in the analysis. Character selection: Unless clearly rejected by homology criteria, characters were not excluded from the phylogenetic analysis, even if they were expected to be homoplastic according to traditional view. In some cases, morphologically similar structures of doubtful homology were tentatively treated as homologous. Homology was tested mainly by similarity and congruence in position, innervation, and microstructure. In contrast, the following characters were eliminated from phylogenetic analysis. (i) Non-discrete, qualitative characters were all excluded. Examples are the depth of pallial cavity, the length of the ctenidial membrane, the length and configuration of the radular sac, all of which show continuous degree of development. (ii) The shell (protoconch) characters were not included in order to evaluate phylogenetic congruence with anatomical characters based on the result of the analysis, (iii) In spite of their potential for resolving a higher phytogeny, all submicroscopic characters were excluded because of the lack of real data in the majority of OTUs. (iv) Even among macroscopic and microscopic characters, some well-known characters were not used in the analysis because of the lack of data for most OTUs. Examples include pericardial and nephridial glands (Fretter, 1990; Ponder and Lindberg, 1997), egg structure (Amio, 1963: figs. 5, 9), cleavage pattern (Biggelaar, 1996; Haszprunar and Biggelaar, 1997), and contents of statocysts (statoliths or statoconia) (Haszprunar, 1988 b). (v) In the female reproductive organ, glands associated with the oviduct and sperm storage sac are not used except for Neritopsina. There seem to be no definite criteria to test their homology across distant higher gastropod taxa (Ponder and Lindberg, 1997). Character coding: Division of character states was discussed in detail prior to the analysis (IV-1). All multistate characters were coded as unordered. No characters were constrained as irreversible and assumed to follow Dollo's parsimony. In the data matrix, unknown or uninvestigated states were scored as "?", and inapplicable states of missing characters or uncertain homology were represented by " - ". Equal weighting was applied to all characters except in the analysis using scaled characters. Search for phylogenetic trees: The data matrix was edited using MacClade ver. 3.04 (Maddison and Maddison, 1992) and analyzed with PAUP ver. 3.1.1 (Phylogenetic Analysis Using Parsimony; Swofford, 1993). The search for phylogenetic trees was carried out by the heuristic search option, but not by branch-and-bound or exhaustive search, because of excessively long calculation time caused by the large data matrix (93 characters for 29 taxa). To increase the likelihood of finding the shortest tree, a random addition sequence of 30 replicates was employed in heuristic analysis with swapping algorithms of tree bisection reconnection (TBR). Steepest descent was not in effect in the analysis. Because the analysis did not produce a single most-parsimonious tree, a strict consensus tree was computed. In further steps, three different methods of analysis, (i) heuristic analysis by scaled weighting, (ii) bootstrap analysis, and (iii) clade decay analysis, were performed to evaluate the robustness of tree topology. In the scaled analysis, 1000 base weights were applied to all characters based on the rescaled consistency index (RCI) using the scale option to assess the effect of multistate characters on tree topology. Character trace: Polarity of character evolution was traced following tree calculation, although some character changes were also determined a priori by outgroup comparison (see Ponder and Lindberg, 1997). Change of character states and synapomorphies for clades were identified in most-parsimonious trees using both ACCTRAN (accelerated transformation) optimization which favors reversals over parallelisms and DELTRAN (delayed transformation) optimization which favors parallelisms over reversals. This procedure was performed using the reconstruction option in PAUP and the character trace option on tree window in MacClade. Taxon names for major gastropod clades: Taxon names for higher gastropod categories in this study followed the schemes of Ponder and War é n (1988) and Ponder and Lindberg (1997). Therefore, Caenogastropoda is equivalent to Cox's (1960) original definition (="Mesogastropoda" + Neogastropoda,= Architaenioglossa + Neotaenioglossa + Neogastropoda). This definition is different from Haszprunar's (1988 a, b) Caenogastropoda in which Architaenioglossa is not included. (5) Summary of Archaeogastropod TaxonomyA review of diagnostic characters and included taxa was presented for archaeogastropod higher taxa at order to family levels in Appendix. It was constructed by literature searches for previous anatomical descriptions in addition to the results of this study. Original literatures were not necessarily cited for the character states verified in this study, but in the case of taxa and characters not treated in descriptive part, references were always shown in the section of included taxa, or directly in each character state. |