CHAPTER 12
Systematic Description of Cryptopecten
|
Subclass Autobranchia Grobben, 1894* Superorder Pteriomorphia Beurlen, 1944* Order Ostreoida Férussac, 1822* Suborder Pectinina Waller, 1978* Superfamily Pectinacea Rafinesque, 1815 Family Pectinidae Rafinesque, 1815 Subfamily Chlamydinae Korobkov, 1960 Genus Cryptopecten Dall, Bartsch and Render, 1938 1938. Cryptopecten Dall, Bartsch and Rehder, Bishop Mus. Bull; vol. 153, p. 93. Type-species. —Cryptopecten alli Dall, Bartsch and Rehder, 1938 [=Pecten (Chlamys) bullatus Dautzenberg and Bavay, 1912]; Hawaii, Philippines and Japan, Late Pliocene to Recent. Historical review. —The generic name Cryptopecten was proposed by Dall, Bartsch and Rehder (1938), who designated C. alli from the sea around Hawaii as the type-species. The original diagnosis runs as follows: The characteristic sculpture of Cryptopecten was partly expressed in the second half of the original diagnosis. Dall, Bartsch and Rehder (1938) probably regarded some other species as belonging to Cryptopecten, because they wrote, "This group appears to have an Indo-Pacific distribution, for we have seen specimens from the Philippines and Fiji, as well as Australia". However, they neither indicated any other specific name as other members of Cryptopecten, nor did they compare it with any other genus. On the other hand, Iredale (1939) proposed genus Corymbichlamys, designating Chlamys corymbiatus Hedley, 1909, from northeastern Australia as its type-species. Hertlein in Cox et al. (1969) regarded Corymbichlamys as congeneric with Argopecten, but I think that it should be treated as a junior synonym of Cryptopecten, because C. corymbiatus is synonymous with Pecten nux Reeve, 1865. Subsequently, Habe (1951, 1977) recognized Cryptopecten as a distinct genus including such Japanese species as Pecten vesiculosus Dunker, 1877, "Pecten tissotii Bernardi, 1858" [=C. bullatus], Pecten nux Reeve, 1865, Pecten inaequivalvis Sowerby, 1887, and Pecten oweni Gregorio, 1936. The generic diagnosis given by him is somewhat different from the original and stresses the stronger convexity of the right valve and the larger size of the anterior wing. Many Japanese malacologists and paleontologists followed him in these generic assignments, but some others did not. For example, Taki and Oyama (1954) and Oyama (1973) regarded Cryptopecten as a subgenus of Aequipecten Fisher, 1886, and Masuda (1962) and Masuda and Noda (1976) referred P. vesiculosus and some related fossil species to Aequipecten. Outside Japan, Hertlein in Cox et al. (1969) treated Cryptopecten as a subgenus of Chlamys, and regarded Gloripallium Iredale, 1939, as a junior synonym of Cryptopecten. He gave the following diagnosis to this subgenus, "Major ribs and interspaces sculptured with radial riblets covered with fluted scales; cardinal crura present". Fatton (1973) also regarded Cryptopecten as a subgenus of Chlamys. Kay (1979) did not accept Cryptopecten as a genus-group taxon, but regarded its type-species, C. alli, as belonging to Chlamys. Thus many different opinions have been presented about the validity and application of the generic (or subgeneric) name of Cryptopecten as well as its limits and taxonomic position. Generally speaking, the classification of Cenozoic and Recent Pectinidae is still in confusion. Pectinids include many conspicuous species which are attractively shaped and beautifully colored, and their taxonomic description began in the later half of the 18th century. Because of insufficient description and poor locality information, the nature of many early described species remains obscure. As listed by Vokes (1967, 1980) and Hertlein in Cox et al. (1969), more than 120 taxa of genus-group have been proposed, but their diagnostic characters and limits as well as relationship to other taxa are not necessarily clear. The same holds true for the subfamilial division of this family. For instance, many genera characterized by deep byssal notch, well-developed ctenolium, relatively tall outline, scarcely gaped disk margin and scaly radial ribs may be grouped in the subfamily Chlamydinae Korobkov, 1960, but their limit and relationship with other subfamilies (e. g., Pectininae) are still very much debatable. In order to settle the classification system of this family, more comprehensive studies should be done on the basis of the materials that exist worldwide and on sound evaluation of taxonomic characters. It goes without saying that studies of fossils are important for the recognition of phylogenetic relationship. In this article discussion is confined to the validity and morphological characteristics of Cryptopecten, but it seems necessary to first clarify its discrimination from other related genera of the Chlamydinae. Among the living Pacific pectinids referred to Cryptopecten, Pecten vesiculosus and Pecten nux share many essential characteristics with the type-species, C. alli [=Pecten (Chlamys) bullatus]. On the other hand, Pecten inaequivalvis is similar to the type-species of Haumea, i. e. H. juddi Dall, Bartsch and Rehder, 1938 [=Pecten loxoides Sowerby, 1882], and should be excluded from the present genus. Specific characters of Pecten oweni are not very clear at present, but specimens in Japan hitherto referred to this species (e. g., Kuroda, 1932; Shikama, 1964) do not have any features of the characteristic Cryptopecten sculpture. The radially striated ribs of P. oweni, so far as these Japanese specimens are concerned, remind me, in fact, of those of Comptopallium Iredale, 1939, and Decatopecten Rüppel in Sowerby, 1839. Pecten phrygium Dall, 1886, from the Mexican Gulf is, as was pointed out by Waller (personal communication, April 21, 1981) and treated by Woodring (1982), undoubtedly another representative of Cryptopecten, because the surface sculpture and many other characters are essentially similar to the type-species. Pecten muscosus Wood, 1828, from the Mexican Gulf, which was assigned to Cryptopecten by Shikama (1964), does not seem to belong to this genus in view of the large wings and spiny (not imbricated) scales on the radial ribs. P. muscosus, I maintain, constitutes another species group together with Pecten acanthodes Dall, 1925, both of which were referred to Aequipecten by Waller (1969, 1973). Ladd (1945) regarded Pecten historionicus Gmelin, 1791, as a doubtful member of Cryptopecten, but this species, as was clarified by Waller (1972a), is an undoubted species of Excellichlamys. Recently Woodring (1982) redescribed Pecten cactaceus Dall, 1898, from the Upper Miocene (or Lower Pliocene) of Panama, regarding it as an early representative of Cryptopecten. As he pointed out, the sculpture of this species is very unique, being characterized by several persistent intercostal threads covered by fine imbricated scales. The hollow structure (see Woodring, 1982, pl. 124, figs. 9, 11) strongly reminds me of that of Phenotype R of Cryptopecten vesiculosus. As a result of my observation of the Panamanian specimens in the National Museum of Natural History, Washington, D. C., however, Pecten cactaceus seems to be considerably different from C. vesiculosus and other representatives of Cryptopecten because of the undeveloped ctenolium, well-developed and persistent intercostal threads, relatively shallow byssal notch, and unusually thin shells. Its generic reference to Cryptopecten is therefore still debatable. At present, it may be adequate that an emended diagnosis of Cryptopecten considers common characteristics of P. vesiculosus, P. nux and P. phrygium in addition to the type-species (P. bullatus) and a few undoubted fossil species. Emended diagnosis of Cryptopecten. —Shell comparatively small-sized for pectinids, with height subequal to length, nearly acline but tending to become slightly prosocline in later growth stages. Convexity of shell variable among species, but right valve commonly more strongly inflated than left, the reverse being the case in early growth stages. Byssal notch moderately deep, provided with several denticles of ctenolium. Wings moderate in size; anterior generally larger than posterior. Anterodorsal margin nearly straight, posterodorsal margin slightly concave, especially in later stages. Apical angle moderately large for pectinids. Disk margin scarcely gaped. Disk suborbicular, ornamented with 12 to 25 strong and simple radial ribs, and in later growth stages with a few fine threads on each interspace as well. Both lateral sides of radial ribs, and sometimes interspaces, covered with fine imbricated scales which enclose narrow hollow chambers. Wings of both valves with several radial ribs without hollow chambers. Early dissoconch marked with delicate Camptonectes-like striae, occurring from much earlier stage in left valve than right and disappearing before shell attains 4 mm in height. Coloration quite variable within each species, but commonly reddish brown. Left valve more darkly pigmented than right. Byssal wing of right valve almost invariably pale. Outer ligament area comparatively thin. Resilial pit small or moderate in size. Comparisons. —One of the striking characteristics of Cryptopecten, as stressed in the original description, lies in the mode of radial ribs, which is characterized by fine imbricated scales covering the sides of the ribs with some hollow space. The radial ribs are quite regular in prominence, neither bifurcated nor inserted except for very early stages. Another conspicuous feature of this genus is the highly allometric growth of shell, which is quite different in the two valves; the shell turns from left-convex to right-convex through equiconvex. These features may not be exclusive, but they are also sometimes observable in certain species of other genera of the Chlamydinae. The taxonomic distinction and validity of Cryptopecten at the generic level, however, are supported by the combination of these characteristics. Some Japanese authors, as noted above, treated Cryptopecten as a subgenus of Aequipecten Fischer, 1886 [type-species: Ostrea opercularis Linnaeus, 1758, monotypy]. The name of Aequipecten seems to have been widely and sometimes inadequately used by many workers for fossil and living pectinids. The type-species of Aequipecten has left-convex shell throughout growth, almost solid radial ribs and widely gaped anterodorsal and posterodorsal margins. In England Aequipecten opercularis is called "Queen scallop", and its anatomical features and mode of life are known in detail (Dakin, 1909; Rees in Cox, 1957). The wide gapes of disk are probably related to swimming habit (Stanley, 1970). On the contrary, Cryptopecten is, so far as I observed in C. vesiculosus, not an active swimmer, and the disk margins are scarcely gaped. Though the suborbicular disk, unequal wings, and regular and simple (neither bifurcated nor inserted) radial ribs of Cryptopecten are similar to those of Aequipecten, the two are quite unlike in the marginal gaping and mode of relative growth. So far as I am aware, there is no true living representative of Aequipecten in the Indo-Pacific. The diagnostic characters and limits of Aequipecten are, however, not necessarily clear. As the result of my preliminary survey, it may be classified into the following three species groups: The first group is, of course, typical Aequipecten. The second group including Aequipecten acanthodes (Dall, 1925), I think, may eventually be generically or subgenerically separable from the first group. The third group includes such right-convex species as Aequipecten. commutatus (Monterosato, 1875) from the Mediterranean and west coast of north Africa and Aequipecten atlanticus (Smith, 1890) from the sea around St. Helena Island. A. commutatus is a particularly impressive species, because it often displays a hollow structure of radial ribs somewhat similar to Cryptopecten, and because some dimorphism is observed in the surface sculpture (Fig. 25). Among several samples of this species in the National Museum of Natural History which I have examined, the sample USNM 764279 from Morocco (off Melilla, 20 fms) is the largest, consisting of 35 complete conjoined valves. In 28 individuals of this sample, each flat-topped radial rib is accompanied by a pair of narrow hollow parts which are covered with fine imbricated scales. On the other hand, hollow structure is completely absent at all in the seven remaining individuals. Although this dimorphic phenomenon should be studied on the basis of more samples and field observation, this may provide a clue to the origin of hollow structure. At the least, it seems to suggest that hollow structure has spread in the populations of A. commutatus through phenotypic substitution. In many respects A. commutatus and its allies appear to be intermediate between typical Aequipecten and Cryptopecten. Hertlein in Cox et al. (1969) regarded Gloripallium Iredale, 1939 [type-species: Ostrea pallium Linnaeus, 1758, original designation] as a junior synonym of Cryptopecten. The tripartite radial ribs of G. pallium may be apparently similar, but the scales on the ribs and interspaces are much coarser and are never imbricated as in the species of Cryptopecten. The ctenolium in young growth stages is exposed on the external surface along the boundary between the byssal wing and disk in every species of Cryptopecten, but is completely concealed by the byssal wing in Gloripallium. Moreover, the disk of Gloripallium is nearly equiconvex, nearly acline throughout growth and similarly pigmented in the two valves. As was pointed out by Waller (1972a), some internal thickening of the wings is observed in Gloripallium, but not in Cryptopecten. Gloripallium and Cryptopecten should be regarded as distinct. Argopecten Monterosato, 1889* [type-species: Pecten solidulus Reeve, 1853 (=Pecten circularis Sowerby, 1835), subsequent designation by Monterosato, 1899] may also be a genus comparable with Cryptopecten. This genus is well represented on the Atlantic coast of the United States, Gulf of Mexico and Caribbean Sea, and a few species are also known in the eastern Pacific from California to Chile (Grau, 1959; Waller, 1969). Fossil Argopecten from the Neogene and Quaternary of North America were thoroughly studied by Waller (1969), and offered an outstanding example of molluscan evolution. The shell outline and convexity of Argopecten are considerably variable, but, as described by Waller (1969) and others, the shell is generally equiconvex and nearly acline throughout growth, and the wings are subequal in size. Consequently, the posterior wing is commonly much larger than that of Cryptopecten. In Argopecten, so far as I am aware, the radial ribs are solid without any hollow structure, and scales are generally undeveloped on the ribs and interspaces. Plagioctenium Dall, 1898 [type-species: Pecten ventricosus Sowerby, 1842 (=Pecten circularis Sowerby, 1835), original designation] is regarded as synonymous with Argopecten. Haumea Dall, Bartsch and Rehder, 1938 [type-species: Haumea juddi Dall, Bartsch and Rehder, 1938 (=Pecten loxoides Sowerby, 1882), original designation] from the shallow waters around the Hawaiian Islands may be related to Argopecten in view of the smoothish radial ribs and interspaces, and undeveloped ctenolium. The right-convex shell throughout growth, very small anterior wing, shallow byssal notch, and decidedly prosocline disk, however, seem to qualify it for designation as a distinct genus. As noted before, Pecten inaequivalvis Sowerby, 1887, from east Asia seems to belong to this genus. Whatever the case, Cryptopecten appears to be unrelated to Haumea. Annachlamys Iredale, 1939 [type-species: Pecten leopardus Reeve, 1853, original designation], mainly from the shallow waters of the western tropical Pacific, clearly differs from Cryptopecten in the shallow byssal notch, subequal wings, and rough growth lamellae on the surface. Though weak denticles of ctenolium are observed in young individuals of a Japanese representative, A. reevei (Adams and Reeve, 1850), this genus is apparently intermediate between the Chlamydinae and the Pectininae. Volachlamys Iredale, 1939 [type-species: Pecten cumingii Reeve, 1853 (=Pecten singaporinus Sowerby, 1842), original designation], from the shallow waters of the western Pacific, is clearly different from Cryptopecten in the much larger size, acutely triangular posterior wing, undeveloped scales on the surface, and solid radial ribs. A Japanese representative, Volachlamys hirasei (Bavay, 1904), shows highly allometric growth and dimorphic phenomenon comparable with the case of Cryptopecten vesiculosus, but these features do not seem to indicate that the two genera are closely related. Excellichlamys Iredale, 1939 [type-species: Pecten spectabilis Reeve, 1853] from the western Pacific and Indian Ocean may be somewhat related to Cryptopecten. As was clearly illustrated by Waller (1972a, pl. 6, figs. 93-96), E. spectabilis shows somewhat similarly imbricated scales which cover the radial ribs with some hollow space. Though E. spectabilis and Excellichlamys histrionica (Gmelin, 1791) from Japan seem to show wide variation in shell outline and surface sculpture, Excellichlamys can be generally distinguished from Cryptopecten by the fewer and often bifurcated radial ribs, undeveloped central solid ridge of each rib, weaker convexity of left valve, and presence of spotted color pattern on the disk. The generic validity and distinctness of Cryptopecten are thus confirmed. As were collectively described above, the surface characters of early dissoconch are nearly the same for the three extant Indo-Pacific species of this genus. Important morphological characters for specific and subspecific discrimination are shell size, convexity of both valves, thickness of test, apical angle, number of radial ribs on disk, size and mode (either opposite or alternate) of imbricated scales covering the sides of radial ribs, shape and sculpture of byssal wing, development of inner shell layer, and presence or absence of dimorphism in surface sculpture. Origin of Cryptopecten. —Little has been studied about the ancestry of genus Cryptopecten. The earliest undoubted representative of this genus so far known is C. yanagawaensis from the lower Middle Miocene of Japan in the Pacific and C. nux from the Lower Miocene of Tanzania in the Indian Ocean. The two species, I presume, may have constituted independent stocks in different regions during Early-Middle Miocene times, but it is now difficult to determine which stock is parental and what their common ancestor is. In this connection, however, it is worthy of notice that there are several Oligocene and Early Miocene pectinids which are considerably similar to the known species of Cryptopecten in general outline and some other shell characters. Such species are especially common in the Oligocene and Miocene of Iran, east Africa and Mediterranean region. Many of them were described under the subgeneric name of Aequipecten by Cox (1927, 1930) and Eames and Cox (1956). "Aequipecten" in Eames and Cox' sense appears to be a somewhat heterogeneous group, to which they referred, for example, Chlamys townsendi and Gloripallium pallium. Nevertheless, some Oligocene and Miocene species from this region may be actually ancestral to the true Recent representatives of Aequipecten in the Mediterranean and eastern Atlantic. It is generally admitted that the marine molluscan fauna of the western Indian Ocean was intimately connected with that of the Mediterranean region until the beginning of Miocene. For example, several species of Aequipecten from the Oligocene of Iran have also been known from Palestine, Italy and south France. After Early Miocene the Tethyan seaway was probably interrupted, and the provinciality of the two sea realms seems to have become clear. On the Indian side, Aequipecten seems to have declined after Miocene, and probably is not represented by any undoubted species in the present sea. Chlamys (Aequipecten) deltoidea Cox, 1927, from the Lower Miocene of Pemba Island,Tanzania, C. (A.) iranica Eames and Cox, 1956, from the Lower Miocene of Iran, and C. (A.) kiwazinensis Eames and Cox, 1956, and C. (A.) pseudotjaringenensis Eames and Cox, 1956, from the Middle Miocene of Iran are rather unlike typical Recent species of Aequipecten, but resemble, though probably only superficially, Cryptopecten yanagawa ensis and C. vesiculosus in outline and number of radial ribs. However, these Miocene species do not show any distinct hollow structure of radial ribs as is commonly seen in Cryptopecten. Further studies of Paleogene and Miocene pectinids in the Tethyan region seem to be necessary in order to determine the phylogenetic relation between Aequipecien and Cryptopecten.
|
 Explanation of Plate 1 (All figures ×2) Figs. 1-6. Cryptopecten bullatus (Dautzenberg and Bavay) Fig. 1. Right valve (USNM 764151), off Waikiki, Hawaii. Fig. 2. Right valve (USNM 764151), the same locality; 2a: external view, 2b: internal view, 2c: anterior view, 2d; dorsal view. Fig. 3. Left valve (USNM 764151), the same locality; 3a: external view, 3b: internal view. Fig. 4. Left valve (USNM 294674), off N. Burias, Philippines ;4a: external view, 4b: internal view. Fig. 5. Living conjoined valves (UMUT RM16002b), sample Hs (3) (B), Hyotanse near Niijima Island; 5a: external view of right valve, 5b: internal view of right valve, 5c: external view of left valve, 5d: internal view of left valve, 5e: anterior view, 5f: dorsal view. Fig. 6. Living conjoined valves (USNM 764152), Tosa Bay, Shikoku; 6a: external view of right valve, 6b: internal view of right valve, 6c: external view of left valve, 6d: internal view of left valve, 6e: anterior view, 6f: dorsal view. |
 Explanation of Plate 2 (All figures × 2, unless otherwise stated) Figs. 1-3. Cryptopecten bullatus (Dautzenberg and Bavay) Fig. 1. Right valve (UMUT CM16001a), sample Kk (5), Late Pleistocene Wan Formation at Kikai Island; 1a: external view, 1b: internal view. Fig. 2. Left valve (UMUT CM16001b), the same sample; 2a: external view, 2b: internal view. Fig. 3. Living conjoined valves (UMUT RM16007a), sample Ks (B), mouth of Kii Strait; 3a: external view of right valve, 3b: external view of left valve, 3c: anterior view, 3d: dorsal view, 3e: ornament on ventral area of right valve showing alternate disposition of imbricated scales (×5, with whitening), 3f: byssal wing (×5, with whitening). Fig. 4. Cryptopecten nux nux (Reeve) Fig. 4. Living conjoined valves (UMUT RM16020), sample An (N), mouth of Ise Bay; 4a: external view of right valve, 4b: internal view of right valve, 4c: external view of left valve, 4d: internal view of left valve, 4e: anterior view, 4f: dorsal view, 4g: ornament on ventral area of right valve showing alternate disposition of almost exfoliated imbricated scales (× 5, with whitening), 4h: byssal wing (×5, with whitening) |
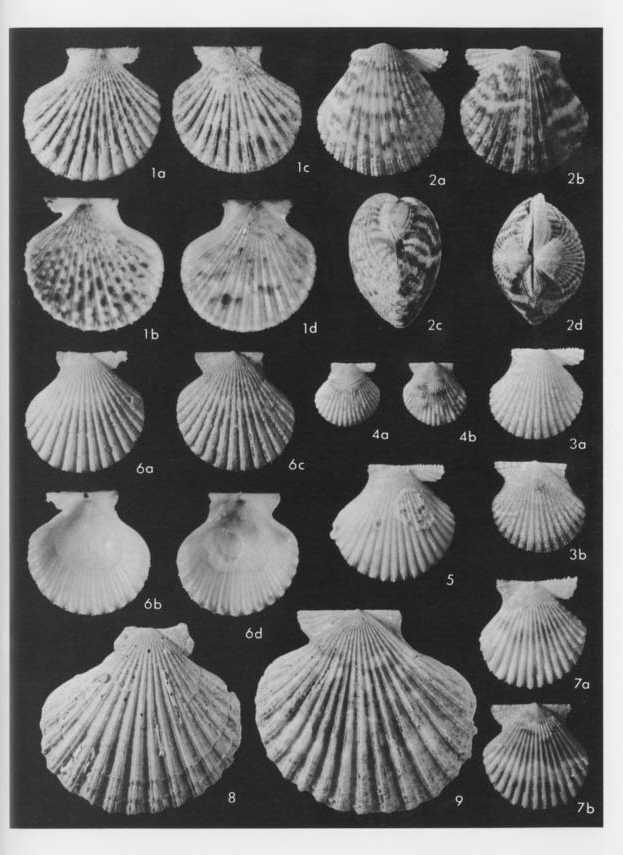 Explanation of Plate 9 Fig. 1. Cryptopecten bullatus (Dautzenberg and Bavay) Fig. 1. Living conjoined valves (USNM 173194), Holotype of Cryptopecten alli Dall, Bartsch and Rehder, 1938; × 1.5; off south coast of Oahu, Hawaii; 1a: external view of right valve, 1b: internal view of right valve, 1c: external view of left valve, 1d: internal view of left valve. Figs. 2. Cryptopecten nux nux (Reeve) Fig. 2. Living conjoined valves (USNM 764157); × 2; Hervey Bay, Queensland; 2a: external view of right valve, 2b: external view of left valve, 2c: anterior view, 2d: dorsal view. Fig. 3. Living conjoined valves (USNM 764164); × 2; Jolo, Philippines; 3a: external view of right valve, 3b: external view of left valve. Fig. 4. Living conjoined valves (USNM 764160); × 2; Itoman, Okinawa, Japan; 4a: external view of right valve, 4b: external view of left valve. Fig. 5. Right valve (USNM 764165); ×2; Al Ghardaqa, Egypt. Figs. 6-9. Cryptopecten phrygium (Dall) Fig. 6. Living conjoined valves (USNM 62352); × 1.5; Mexican Gulf; 6a: external view of right valve, 6b: internal view of right valve, 6c: external view of left valve, 6d: internal view of left valve. Fig. 7. Living conjoined valves (USNM 764544); × 2; off Hillsboro, Florida; 7a: external view of right valve, 7b: external view of left valve. Fig. 8. Right valve (USNM 694425); × 1.2; Mexican Gulf. Fig. 9. Left valve (USNM 764542); × 1.2; off southwest Louisiana, Mexican Gulf. |
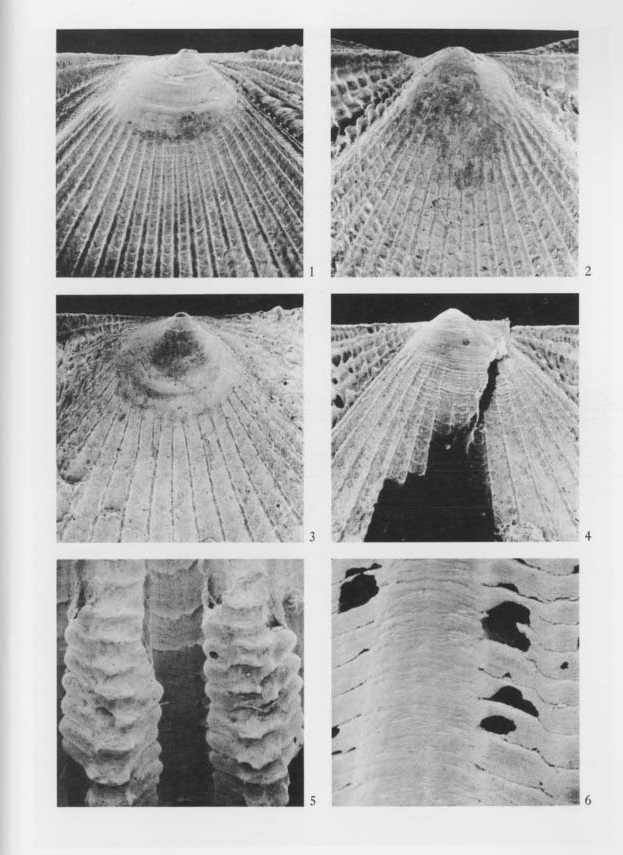
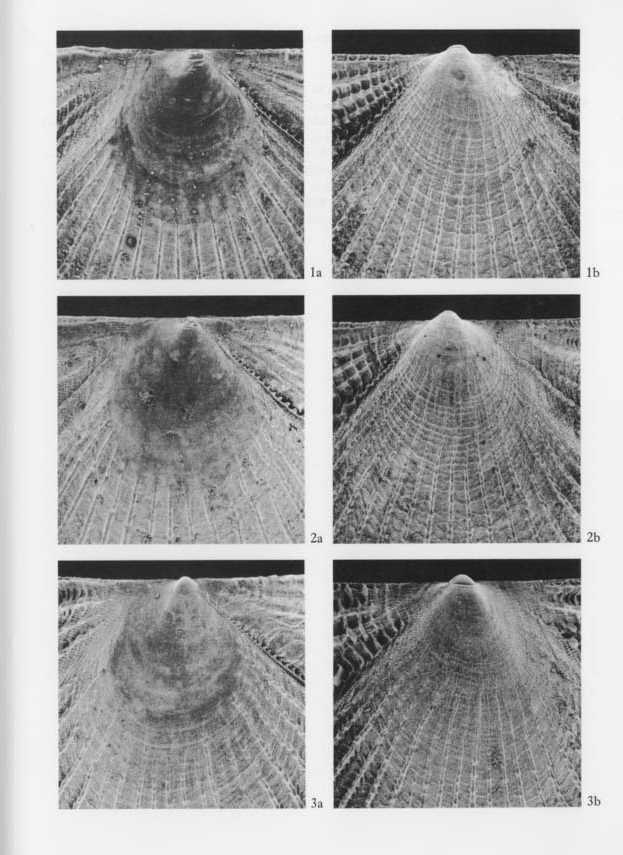 Explanation of Plate 10 (SEM photographs, all figures × 29, 5 KV) Figs. 1, 2. Cryptopecten vesiciilosus vesiculosus (Dunker) Fig, 1. Living conjoined valves (UMUT RM16059a), Phenotype Q,, sample Hy, off southwestern coast of Miura Peninsula; 1a: umbonal area of right valve, 1b: umbonal area of left valve. Fig. 2. Living conjoined valves (UMUT RM16059b), Phenotype R, the same sample; 2a: umbonal area of right valve, 2b: umbonal area of left valve. Fig. 3. Cryptopecten bullatus (Dautzenberg and Bavay) Fig. 3. Living conjoined valves (UMUT RM16002a), sample Hs (3) (A), Hyôtanse near Niijima Island; 3a: umbonal area of right valve, 3b: umbonal area of left valve. |
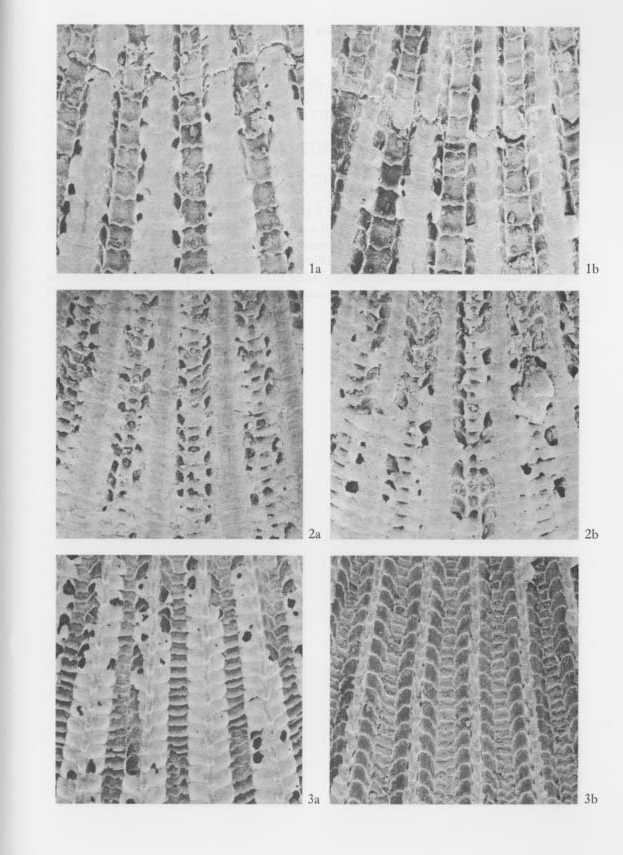 Explanation of Plate 11 (SEM photographs, all figures × 29, 5 KV) Figs. 1, 2. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 1. Living conjoined valves (UMUT RM16059a), Phenotype Q,, the same specimen as Pl. 10, Fig. 1; 1a: middle part of disk of right valve, 1b: middle part of disk of left valve. Fig. 2. Living conjoined valves (UMUT RM16059b), Phenotype R, the same specimen as Pl. 10, Fig. 2; 2a: middle part of disk of right valve, 2b: middle part of disk of left valve. Fig. 3. Cryptopecten bullatus (Dautzenberg and Bavay) Fig. 3. Living conjoined valves (UMUT RM16002a), the same specimen as Pl. 10, Fig. 3; 3a: middle part of disk of right valve, 3b: middle part of disk of left valve. |



?1882. Pecten tissoti [sic] Bernardi: Dunker, Index Molluscorum maris Japonici, p. 241. [non Pecten tissotii Bernardi, 1858]
1902. Pecten tissoti [sic] Bernardi: Yoshihara, Zoo/. Mag. Tokyo, vol. 14, p. 358, pl. 5, fig. 23. [non Pecton tissotii Bernardi, 1858]
1912. Pecten (Chlamys) bullatus Dautzenberg and Bavey, Les Lamell. Expéd. Siboga, System., I. Pectinidés, p. 143, pl. 27, figs. 1, 2.
1932. Chlamys (Aequipecten) tissotii (Bernardi): Kuroda, Venus, vol. 3, no. 2, p. app. 95. [non Pecten tissotii Bernardi, 1858]
1938. Cryptopecten alli Dall, Bartsch and Render, Bishop Mus. Bull., vol. 153, p. 93, pl. 23, figs. 1-4, 7.
1951. Cryptopecten tissotii (Bernardi): Habe, Genera of Japanese Shells, p. 77. [Non Pecten tissotii Bernardi, 1858]
1958. Cryptopecten tissotii (Bernardi): Habe, Publ. Seto Mar. Biol. Lab., vol. 6, no. 3, p. 265. [non Pecten tissotii Bernardi, 1858]
1961. Cryptopecten tissotii (Bernardi): Habe, Coloured Illustrations of the Shells of Japan (II), p. 118, pl. 53, fig. 8. [non Pecten tissotii Bernardi, 1858]
1964. Cryptopecten tissotii (Bernardi): Habe, Shells of the Western Pacific in Color, vol. 2, p. 174, pl. 53, fig. 8. [non Pecten tissotii Barnardi, 1858]
1969 Chlamys (Cryptopecten) alli (Dall, Bartsch and Render): Hertlein in Cox et al., Treatise on Invertebrate Paleontology, pt. N, vol. 1, p. N357, fig. C79-la, b.
1972. Cryptopecten "tissotii (Bernardi)": Okutani, Bull. Tokai Reg. Fish. Res. Lab., no. 72, p. 113, fig. 62. [non Pecton tissotii Bernardi, 1858]
1975. Cryptopecten tissotii (Bernardi): Okutani and Habe, Mollusca II chiefly from Japan, p. 91, 4 figs., p. 256. [non Pecten tissotii Bernardi, 1858]
1977. Cryptopecten tissotii (Bernardi): Habe, Systematics of Mollusca in Japan, p. 84. [non Pecten tissotii Bernardi, 1858]
1979. Chlamys alli (Dall, Bartsch and Render): Kay, Bishop Mus. Spec. Publ. vol. 64, no. 4, p. 524, fig. 168A.
1980. Aequipecten sp.: Noda, Sci. Rep. Inst. Geosci. Univ. Tsukuba, sec. B, vol. 1, p. 82, pl. 12, fig. 21.
1981. Cryptopecten alli Dall, Bartsch and Rehder: Poutiers, Mem. O. R. S. T. O. M., no. 91, p. 326, 332.
1982. Cryptopecten alli Dall, Bartsch and Rehder: Hayami, Venus, vol. 41, p. 234.

Type. —The holotype of Pecten (Chlamys) bullatus is a conjoined specimen collected by the Siboga expedition at Station 105 off Sulu Archipelago (6°08'N, 121°19'E) in 275 meters. 13.9 mm long, 13.3 mm high, 3.0 mm thick. It is now preserved in the Amsterdam Zoological Museum. The holotype of Cryptopecten alli (USNM 173194) is a conjoined specimen collected by the R/V Albatross of the U.S. Bureau of Fisheries at Station 3811 off the south coast of Oahu Island in 238-252 fathoms on coarse sand and rocky bottom. 22.8 mm long, 22.1 mm high, 5.3 mm thick.
Material. —Specimens used for the present description and discussions are indicated in the list of examined samples.
Diagnosis. —Medium- or small-sized species of Cryptopecten characterized by thin test,weak convexity of both valves, low outline, large apical angle and 18-23 radial ribs which have fine alternate imbricated scales covering both sides of a narrow central ridge and are strongly impressed on internal surface owing to scarcely deposited inner shell layer.
Description. —Shell rarely exceeding 25 mm in length and height, inequivalve, inequilateral, acline in young stage but becoming considerably prosocline in adult stage, left-convex in young but equiconvex or even right-convex in adult; height negatively allometric to length, nearly equal to length in young but invariably less than length in adult; thickness positively allometric to length (and height) in right valve but nearly isometric in left; form ratio T/L scarcely exceeding 0.24 even in adult right valve and 0.18 in adult left valve; length of hinge line moderate, negatively allometric to overall length; test very thin; apical angle of disk about 110 degrees; wings moderate in size, anterior one slightly larger than posterior; pre-umbonal dorsal margin of right valve slightly elevated above hinge axis; posterodorsal margin of disk a little concave and much longer than anterodorsal in adult, while both nearly straight and subequal in length in young; byssal wing moderate in breadth; byssal notch moderate in depth, with four or five exposed denticles of ctenolium and relatively wide fasciole area; anterodorsal and posterodorsal margins of disk scarcely gaped between valves.
Outer surface of disk ornamented with 18-23 radial ribs; each rib consisting of a slender central solid ridge and hollow parts on its sides; solid ridge very sharp, occupying about one-fourth of a rib in breadth; both hollow parts separated into numerous minute chambers and covered with fine imbricated scales numbering about four to six per 1 mm on middle-ventral surface of adult shell; scales on the sides of central ridge very regularly alternate, though oppositely disposed on sides of each interval between ribs; interspace commonly narrower than rib, ornamented with a few fine radial threads only in adult stage, also marked with obliquely inclined scales which occur quite independently from and generally more densely spaced than those on ribs; stepwise growth ring(s) occasionally occur in adult stage; ornamentation on disk essentially the same on both valves; byssal wing commonly possesses five radial ribs which have many spiny scales and become weaker downwards; other wings also have several radial ribs of variable strength.
Coloration variable, but commonly reddish brown and mottled with irregular (oblique or zigzag) pale bands; contrast between parti-colored bands more remarkable in left valve than in right; different pigmentation in two valves also well recognized from inner surface; umbonal area and byssal wing always pale in color.
Inner surface vitreous in luster and strongly plicated in accordance with external sculpture except for umbonal area, due to poor development of inner shell layer; musculature very weakly impressed; resilial insertion relatively small; cardinal crura undeveloped.
Remarks. —Since Yoshihara's (1902) description of several Japanese specimens, many Japanese authors have erroneously called the present species Pecten tissotii Bernardi, 1858. This confusion seems to have arisen from Dunker (1882), who assigned a Japanese specimen (not illustrated) to P. tissoti [sic] Bernardi with a query. In the original description of P. tissotii, Bernardi (1858) did not give any indication about the locality, though Pecten swiftii Bernardi, 1858, described in the same article as a collection from the Gulf of Tartary, is a well-known species from north Japan. The original figure of P. tissotii (Bernardi, 1858, pl. 1, fig. 2) is at a glace similar to the present species in general outline, but clearly differs from it in the larger number of radial ribs, more sigmoidal frontal margin of left anterior wing, and scarcely impressed radial ribs on the inner surface (suggesting better-developed inner shell layer).
Recently Poutiers (1981) identified a solitary valve sampled by the Musorstom expedition from Lubang of the Philippines (217-230 m in depth) with Cryptopecten alli. He said, "The species described and figured by Habe (1964) under the name of 'Cryptopecten tissotii (Bernardi)' does not correspond to the true Pecten tissotii of Bernardi (J. Conchyl. 1858, p. 91, pl. 1, fig. 2), the type of which, preserved in the Laboratoire de Malacologie du Museum d'Histoire naturelle de Paris, was examined by the present author" [translated from French]. Moreover, according to Dr. T. R. Waller's personal communication (May 27, 1981), the type specimen of P. tissotii in Paris seems to belong to Aequipecten flabellus (Gmelin, 1791), which is actually an eastern Atlantic species.
All of the examined specimens from various stations around Japan and the Philippines agree well in every essential character with the Hawaiian specimens of Cryptopecten alli, including the holotype. Thus I once applied the specific name of C. alli to the species under consideration (Hayami, 1982). Subsequently, however, I recognized that the illustrated specimen (holotype) of Pecten (Chlamys) bullatus Dautzenberg and Bavay, 1912, in Amsterdam is nothing but an immature individual of the same species. According to Dr. T. R. Waller's photographs, this specimen shows weak convexity of both valves and about 20 radial ribs, each of which consists of a narrow central ridge and a pair of hollow parts covered with regularly alternate imbricated scales; that is to say, every diagnostic character of C. alli is exhibited in this specimen. Consequently, C. alli should be .regarded as synonymous with C. bullatus.
The geographic variation of C. bullatus does not seem to be wide; none of the samples from Hawaii, the Philippines or Japan shows any conspicuous local characteristics. In the National Museum of Natural History, however, there is an aberrant small sample (USNM 773983) from Nasca Ridge in the eastern Pacific (25°44'S, 85°25'W, 228 m). All the specimens (three right valves) in this sample are nearly white, and this is certainly not due to abrasion. Because such albinism is rarely met with in samples from the central and northwestern Pacific, this sample may represent an isolated local population.
Fossils of C. bullatus seem to be rare. The Late Pleistocene fossil sample Kk (B) from the Wan Formation of Kikai Island is composed of somewhat smaller specimens with slightly more numerous radial ribs than Recent specimens. The structure of radial ribs and other characteristics are, however, essentially the same, and subspecific distinction may be unnecessary. A small left valve from the Late Pliocene Shinzato Formation of Okinawa, which was illustrated under the name of Aequipecten sp. by Noda (1980), can be regarded as an immature individual of this species.
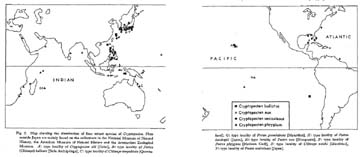
Distribution. —The present species is widely distributed on the lower sublittoral and upper bathyal sandy substrates in the central and northwestern Pacific. In addition to the type locality and several other stations around the Philippines, it has been known from a number of stations around the Hawaiian and Japanese Islands and a station on Nasca Ridge in the eastern Pacific, as is partly recorded in the list of examined samples. Fossil occurrence of C. bullatus is so far restricted to some Pliocene and Pleistocene deposits of the Ryukyu Islands. Late Pliocene (ca. 2.0 Ma) to Recent.
Cryptopecten nux (Reeve)
Diagnosis. —Tumid, smallest species of Cryptopecten characterized by relatively strong inflation of both valves, large anterior wing, 18-22 apparently tripartite radial ribs, regularly alternate fine imbricated scales on radial ribs and moderately thick test.
Remarks. —C. nux is similar to C. bullatus and different from C. vesiculosus in the small shell size, large number of radial ribs, slender central ridge of each rib, and regularly alternate disposition of imbricated scales. However, the convexity of both valves is generally much stronger in comparison with the other two species (see Figs. 9, 10). The inner shell layer is poorly developed in C. bullatus but attains considerable thickness in the present species as well as in C. vesiculosus.
Dr. T. R. Waller suggested to me (oral communication) the possibility that Pecten nux Reeve, 1853, together with several subsequently proposed nominal species, may fall into a junior synonym of Pecten bernardi Philippi, 1851, because one of the "type-specimens" of P. bernardi with no locality data, which is preserved in the British Museum (Natural History) (BM (NH) 1923. 7.13.7), shares essentially the same morphological characters with the illustrated syntype of P. nux. As he commented, however, some ambiguity still remains as to the type identity, because Philippi's original description was brief and not accompanied by any illustrations. For the time being I regard P. nux as the earliest undoubted name for the species under consideration.
Among several fossil samples of this species from Japan, the Late Pleistocene sample Sm (N shows peculiar morphological features deserving a subspecific distinction. Consequently, all the other fossil and Recent samples treated here are provisionally assigned to the nominate subspecies, Cryptopecten nux nux.
Geographic variation. —In addition to several fossil and Recent samples from central and south Japan, I have examined numerous well-documented Recent samples of this species from extensive areas of the Indo-Pacific which are preserved in the National Museum of Natural History, Washington D. C. These samples often differ from one another in such characters as shell size, convexity, obliquity, thickness of test, and shape and sculpture of byssal wing. The difference must be partly due to the age heterogeneity but is mostly attributable to geographic variation. The average number and tripartite appearance of radial ribs, however, hardly vary at all, It is my impression that the center of distribution of this species is in the Philippine Islands, though it seems to be also common in some areas of Melanesia, Polynesia, Queensland and the Indian Ocean.
Most of the specimens from the Philippines are characterized by relatively small and right-convex shell and moderate thickness of test. The fossil and Recent samples from various localities in south Japan are not much different from these specimens in shell morphology. On the other hand, the observed specimens from the Marquesas Islands, the type locality of P. mux, as well as those from New Britain, show thicker test and more equiconvex shell. The material from Queensland, as exemplified by the specimen USNM 764157 (Plate 9, Fig. 2) and the illustrated syntype of Chlamys corymbiatus, possesses thick test and occasionally weak tubercles on the central solid ridge of each radial rib, which are seemingly absent in the specimens from other regions. Generally speaking, the specimens from the Indian Ocean appear to be more weakly inflated than those from the Philippines and many other areas of the Pacific. The holotype of Chlamys smithi Sowerby, 1908, from Mauritius Island, seems to exemplify the Indian Ocean form. Moreover, the examined sample (USNM 764165) from the northern part of the Red Sea (eastern coast of Egypt) is unique for the nearly equiconvex and unusually weakly inflated valves (Plate 9, Fig. 5).
The geographic variation of C. mux is thus considerably wide. If subspecific division were applied to this species, the trivial names corymbiatus and smithi (or guendolenae) would be available for the populations of Queensland and the main part of the Indian Ocean, respectively. The morphological change in these areas, however, may be gradational, and all the observed samples except a few Philippine ones are too small in sample size to recognize dines or to detect significant morphological difference between local populations.
Cryptopecten mux nux (Reeve)
Pl. 2, Fig. 4; Pl. 3, Figs. 1, 2; Pl. 9, Figs. 2-5; Pl. 12, Figs. 1, 2
 Explanation of Plate 2 (All figures × 2, unless otherwise stated) Figs. 1-3. Cryptopecten bullatus (Dautzenberg and Bavay) Fig. 1. Right valve (UMUT CM16001a), sample Kk (5), Late Pleistocene Wan Formation at Kikai Island; 1a: external view, 1b: internal view. Fig. 2. Left valve (UMUT CM16001b), the same sample; 2a: external view, 2b: internal view. Fig. 3. Living conjoined valves (UMUT RM16007a), sample Ks (B), mouth of Kii Strait; 3a: external view of right valve, 3b: external view of left valve, 3c: anterior view, 3d: dorsal view, 3e: ornament on ventral area of right valve showing alternate disposition of imbricated scales (×5, with whitening), 3f: byssal wing (×5, with whitening). Fig. 4. Cryptopecten nux nux (Reeve) Fig. 4. Living conjoined valves (UMUT RM16020), sample An (N), mouth of Ise Bay; 4a: external view of right valve, 4b: internal view of right valve, 4c: external view of left valve, 4d: internal view of left valve, 4e: anterior view, 4f: dorsal view, 4g: ornament on ventral area of right valve showing alternate disposition of almost exfoliated imbricated scales (× 5, with whitening), 4h: byssal wing (×5, with whitening) |
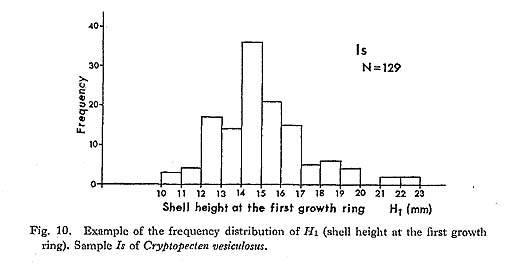
 Explanation of Plate 3 (All figures ×2, unless otherwise stated) Figs. 1-2. Cryptopecten nux nux (Reeve) Fig. 1. Right valve (UMUT CM16015c), sample Kk (N), Late Pleistocene Wan Formation at Kikai Island; 1a: external view, 1b: internal view, 1c: anterior view, 1d: dorsal view. Fig. 2. Left valve (UMUT CM16015d), the same sample; 2a: external view, 2b: internal view, 2c: anterior view, 2d: dorsal view. Fig, 3-4: Cryptopecten nux sematensis (Oyama) Fig. 3. Right valve (UMUT CM21561), Holotype, Yokoyama's specimen (1922, pl. 15, fig. 1), "Shito" (probably identical with the locality of sample Sm (N)); 3a: external view, 3b: internal view, 3c: anterior view, 3d: dorsal view. Fig. 4. Left valve (UMUT CM16180a), sample Sm (N), Late Pleistocene Yabu Formation at Ochishimoshinden; 4a: external view, 4b: internal view, 4c: anterior view, 4d: dorsal view. Fig. 5. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 5. Population sample (UMUT RM16138), sample Is, mouth of Ise Bay; Left: individuals belonging to Phenotype R, Right: individuals belonging to Phenotype Q. (×0.8) |
 Explanation of Plate 9 Fig. 1. Cryptopecten bullatus (Dautzenberg and Bavay) Fig. 1. Living conjoined valves (USNM 173194), Holotype of Cryptopecten alli Dall, Bartsch and Rehder, 1938; × 1.5; off south coast of Oahu, Hawaii; 1a: external view of right valve, 1b: internal view of right valve, 1c: external view of left valve, 1d: internal view of left valve. Figs. 2. Cryptopecten nux nux (Reeve) Fig. 2. Living conjoined valves (USNM 764157); × 2; Hervey Bay, Queensland; 2a: external view of right valve, 2b: external view of left valve, 2c: anterior view, 2d: dorsal view. Fig. 3. Living conjoined valves (USNM 764164); × 2; Jolo, Philippines; 3a: external view of right valve, 3b: external view of left valve. Fig. 4. Living conjoined valves (USNM 764160); × 2; Itoman, Okinawa, Japan; 4a: external view of right valve, 4b: external view of left valve. Fig. 5. Right valve (USNM 764165); ×2; Al Ghardaqa, Egypt. Figs. 6-9. Cryptopecten phrygium (Dall) Fig. 6. Living conjoined valves (USNM 62352); × 1.5; Mexican Gulf; 6a: external view of right valve, 6b: internal view of right valve, 6c: external view of left valve, 6d: internal view of left valve. Fig. 7. Living conjoined valves (USNM 764544); × 2; off Hillsboro, Florida; 7a: external view of right valve, 7b: external view of left valve. Fig. 8. Right valve (USNM 694425); × 1.2; Mexican Gulf. Fig. 9. Left valve (USNM 764542); × 1.2; off southwest Louisiana, Mexican Gulf. |
 Explanation of Plate 12 (SEM photographs, all figures ×29, 5 KV) Figs. 1, 2. Cryptopecten nux nux (Reeve) Fig. 1. Umbonal area of right valve (UMUT CM16015a), sample Kk (N), Late Pleistocene Wan Formation at Kikai Island. Fig. 2. Umbonal area of left valve (UMUT CM1601Sb), the same sample. Figs. 3-5. Cryptopecten spinosus sp. nov. Fig. 3. Umbonal area of right valve (UMUT CM16170a), sample Kk (S), Late Pleistocene Wan Formation at Kikai Island. Fig. 4. Umbonal area of left valve (UMUT CM16170b), the same sample. Fig. 5. Radial ribs on ventral area of right valve (UMUT CM16170a), the same specimen as Pl. 12, Fig. 3, showing oppositely disposed imbricated scales. Fig. 6. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 6. Radial rib on middle-ventral area of adult left valve (UMUT RM16061a), Phenotype R, sample Jg (2), off Jôgashima Islet, Sagami Bay, showing oppositely disposed imbricated scales. |
1853. Pecten coruscans Hinds: Reeve, Conchologia Iconica, vol. 8, pl. 32, fig. 143. [non Pecten coruscans Hinds, 1845]
1853. Pecten nux Reeve, Conchologia Iconica, vol. 8, errata.
1888. Pecten hastingsii Melvill, Jour. Conchology, vol. 5, p. 279, pl. 2, fig. 7.
1888. Pecten guendolenae Melvill, Jour. Conchology, vol. 5, p. 279, pl. 2, fig. 6.
1888. Pecten nux Reeve: Küster and Kobelt in Martini and Chemnitz, System. Conch. Cab., vol. 7, pt. 2, p. 163, pl. 45, figs. 5-8.
1908. Chlamys smithi Sowerby, Proc. Malacol. Soc. London, vol. 8, p. 18, pl, 1, figs. 6, 7.
1909. Chlamys corymbiatus Hedley, Proc. Linn. Soc. New South Wales, vol. 34, p. 423, pl. 36, figs. 1-4.
1912. Pecten (Aequipecten) vesiculosus Dunker: Dautzenberg and Bavay, Les Lamell. Expéd.
Siboga, System., 1. Pectinidés, p. 148. [non Pecten vesiculosus Dunker, 1877]
1930. Chlamys (Aequipecten) nux (Reeve): Cox, Monogr. Geol. Dept. Hunterian Mus., Glasgow Univ., vol. 4, p. 124, pl. 14, fig. 11.
1933. Pecten (Aequipecten) nux Reeve: Nomura, Sci. Rep. Tohoku Imp. Univ., ser. 2, vol. 16, no. l,p. 55.
1934. Pecten (Aequipecten) nux Reeve: Nomura and Zinbo, Sci. Rep. Tohoku Imp. Univ., ser. 2, vol. 16, no. 2, p. 117 (pars).
1934. Pecten (Aequipecten) kikaiensis Nomura and Zinbo, Sci. Rep. Tohoku Imp. Univ., ser. 2, vol. 16, no. 2, p. 153, pl. 5, fig. 9a, b.
1939. Corymbichlamys corymbiatus (Hedley): Iredale, Brit. Mus. (Nat. Hist.) Sci. Rep., vol. 5 (Mollusca pt. 1), p. 368.
1951. Cryptopecten nux (Reeve): Habe, Genera of Japanese Shells, p. 77.
1956. Chlamys (Aequipecten) nux (Reeve): Eames and Cox, Proc. Malacol. Soc. London, vol. 32, p. 43.
1961. Cryptopecten nux (Reeve): Habe, Coloured Illustrations of the Shells of Japan (II), p. 118, pl. 53, fig. 9.
1964. Chlamys (Cryptopecten) nux (Reeve): Shikama, Selected Shells of the World II, p. 50, fig. 93.
1964. Cryptopecten nux (Reeve): Habe, Shells of the Western Pacific in Color, vol. 2, p. 174, pl. 53, fig. 9.
1977. Cryptopecten nux (Reeve): Habe, Systematics of Mollusca in Japan, p. 84.
1977. Cryptopecten kikaiensis (Nomura and Zinbo): Habe, Systematics of Mollusca in Japan, p. 84.
1977. Cryptopecten hastingsii (Melvill): Habe, Systematics of Mollusca in Japan, p. 85.
1982. Cryptopecten nux (Reeve): Hayami, Venus, vol. 41, p. 235.
Type. —Reeve's illustrated syntype (BM (NH) 1950. 11.14.52), which was erroneously named "Pecten coruscans" in the original text but subsequently corrected in the index and errata, is a conjoined specimen from Marquesas Island of Polynesia. It is preserved in the British Museum (Natural History). ca. 15 mm long, 14 mm high, 8 mm thick. The holotype of Pecten hastingsii from Japan (ca. 25 mm long, 24 mm high, 14 mm thick) and the holotype of Pecten guendolenae from Mauritius (ca. 15 mm long, 14 mm high, 7 mm thick) are conjoined specimens preserved in the Cardiff Museum (Tomlin Collection). The holotype of Chlamys smithi from Mauritius (BM (NH) 1908.5.30.63) (ca. 16 mm long, 16 mm high, 7 mm thick) is a conjoined specimen preserved in the British Museum (Natural History). The syntypes of Chlamys corymbiatus are said to have been presented to the Australian Museum. The holotype of Pecten (Aequipecten) kikaiensis is a right valve (IGPS no. 50357) from the Late Pleistocene Wan Formation at Kamikatetsu of Kikai Island, south Japan, which is preserved in the Institute of Geology and Palaeon tology, Tohoku University.
Material. —Specimens used for the following description and discussions are indicated in the list of examined samples.
Diagnosis. —Subspecies of C. nux, possessing variably but usually strongly inflated valves and narrowly elongated byssal wing with prominent radial ribs and often with spiny scales.
Description. —Shell small, rarely exceeding 20 mm in length and height, highly in equivalve, nearly acline or very slightly prosocline, more or less right-convex except for very early growth stages; height negatively allometric to length, commonly subequal to length in young stages but becoming considerably smaller than length with growth; thickness nearly isometric to length in each valve; form ratio T/L greatly variable even within a sample, ranging 0.23-0.37 in adult right valves and 0.17-0.26 in adult left valves; hinge line moderate or comparatively long, negatively allometric to overall length; test moderate but somewhat variable in thickness; apical angle of disk 100-105 degrees; anterior wing more than twice as long as posterior; byssal wing generally slender, with elevated dorsal margin above hinge axis; anterodorsal and posterodorsal margins of disk nearly straight, subequal in length, scarcely gaped between valves; byssal notch moderate in depth, with four or five exposed denticles of ctenolium and fasciole area of moderate width.
Outer surface of disk ornamented with 18-22 radial ribs, each of which consists of a solid, roof-shaped central ridge and a pair of hollow parts on its sides covered by fine alternate imbricated scales; accordingly, if scales are exfoliated or removed, radial ribs look regularly tripartite; imbricated scales numbering eight to nine per 1 mm on middle ventral surface of adult shell; interspace of ribs as wide as rib, marked with short erect scales, which are independent from and generally more distantly spaced than imbricated scales on ribs; intercostal radial threads commonly absent but rarely observed in some adult individuals; growth ring(s), if present, very weak; disk sculpture of both valves essentially similar; byssal wing generally possesses four strong radial ribs on which several spiny scales frequently occur; other wings also ornamented with a few radial riblets.
Coloration variable, sometimes monotonously yellow but more commonly reddish brown in ground color with irregular pale bands and spots; left valve generally more darkly pigmented than right, not only externally but also internally; byssal wing always pale in color.
Internal surface somewhat shiny, strongly plicated in accordance with radial ribs, but umbonal to middle part much flattened by deposition of inner shell layer; musculature weakly impressed; resilial insertion moderate in size; cardinal crura developed but not very strong.
Remarks. —This subspecies seems to include almost all the specimens hitherto described under the names of Pecten nux and Chlamys corymbiatus and also the type materials of Pecten hastingsii, Pecten guendolenae, Chlamys smithi and Pecten (Aequipecten) kikaiensis.
In spite of an extensive geographic distribution in the Indo-Pacific, the present subspecies seems to be comparatively rare in the seas around the Japanese Islands: every Recent sample consists of one or a few individuals. Only the sample Kk (N) from the Late Pleistocene of Kikai Island is composed of a large number of individuals, allowing quantitative examination of intrapopulational variation and relative growth (Tables 3, 4, 6-9). At the same locality two other species of Cryptopecten, i. e., C. bullatus and C. spinosus, occur in association, and they are clearly distinguishable from the present subspecies. As suggested from this sample as well as such samples as Bh (N), USNM 230228, 230314 and 292450 from the Philippines, the intrapopulational variation of shell outline and convexity is considerably wide; the right valve is generally characterized by strong convexity, but the form ratio T/L is quite variable, as indicated by the relatively low value of correlation coefficient between log L and log T (or log C).
Besides the sample Kk (N), C. nux nux is represented by several small samples of various geological ages from the Ryukyu Islands (samples Mk (N), Ik (N) and Ob (N)). Though their morphometric comparison is still difficult owing to their small sample size, essential morphological characters including the mode and number of radial ribs appear almost unchanged throughout the geological period.
Neogene fossils of C. nux are are considerably well represented in the tropical coastal region of east Africa. Cox (1930) and Eames and Cox (1956) recorded occurrences of this species from the Lower Miocene at several localities in Zanzibar and Pemba Islands in Tanzania, and also from the Pliocene near Mombasa in Kenya. Although I have had no opportunity to observe the African specimens, it is quite likely that the range of this species extends down into the Early Miocene. For the time being I regard these fossils as belonging to C. nux nux.
Distribution. —This subspecies is widely distributed in the tropical and subtropical seas of the central-west Pacific and the Indian Ocean. Although dead shells have been dredged from outer sublittoral and bathyal bottoms, the hitherto known records of living shells are almost entirely confined to the inner sublittoral zone. Recent specimens have been known from Polynesia (especially Marquesas and Tahiti), Melanesia, Micronesia, north east Australia, Indonesia, Singapore, the Philippines, the South China Sea, Formosa, south Japan, Bengal Bay, Andaman, Oman, Seychelles, Mauritius, Madagascar, Mozam bique, east coast of South Africa, and the Red Sea. There is no undoubted record of occurrence in the Japan Sea. Fossils of this subspecies occur from the Lower Miocene of east Tanzania (Eames and Cox, 1956), the Pliocene of east Kenya (Cox, 1930), and the Pliocene Byoritsu Formation of Formosa (Nomura, 1933) in addition to the Pliocene and Pleistocene in central and south Japan (see the list of examined samples). Early Miocene to Recent.
Cryptopecten nux sematensis (Oyama)
Pl. 3, Figs. 3, 4


1922. Pecten tissoti [sic] Bernardi: Yokoyama, Jour. Coll. Sci. Imp. Univ. Tokyo, vol. 44, art. 1, p. 182, pl. 15, figs. 1, 2. [non Pecten tissotii Bernardi, 1858]
1954. Aequipecten (Cryptopecten) sematensis Oyama in Taki and Oyama, Palaeont. Soc, Japan, Spec. Papers, no. 2, pl. 35, figs. 1, 2.
1973. Aequipecten (Cryptopecten) sematensis Oyama: Oyama, Palaeont. Soc. Japan, Spec. Papers, no. 17, p. 85, pl. 34, figs. 9a, b, 10a, b.
1976. Aequipecten vesiculosus (Dunker): Masuda and Noda, Check List and Bibliography of the Tertiary and Quaternary Mollusca of Japan, p. 8 (only), [non Pecten vesiculosus Dunker, 1877]
Type. —The holotype is a nearly complete right valve (UMUT CM21561) which was described by Yokoyama (1922, pl. 15, fig. 1) as "Pecten tissoti" and designated by Oyama in Taki and Oyama (1954) as the holotype of Aequipecten (Cryptopecten) semafensis. It occurred from "the Upper Musashino at Shito", which is certainly identical with the upper part of the Yabu Formation at Ochishimoshinden of Semata, Toki Town, Chiba Prefecture (35°31.5'N, 140°13.7'E), where the sample Sm (N) was newly obtained. 15,2 mm long, 17.1 mm high, 6.2 mm thick.
Material. —In addition to the holotype, twelve specimens of the sample Sm (N) are concerned with the following description and discussions.
Diagnosis. —Subspecies of C. nux, characterized by relatively tall outline, very strongly inflated right and left valves, and short and broad byssal wing without any prominent spiny scales.
Description. —Shell small, rarely exceeding 18 mm in length and height, nearly acline throughout growth, right-convex except for very young stages; height isometric or slightly negatively allometric to length, commonly exceeding length; thickness decidedly positively allometric to length (and height) in each valve; form ratio T/L as large as 0.36- 0.41 in adult right valve and 0.26-0.29 in adult left valve; apical angle of disk about 100- 105 degrees; test relatively thick; byssal wing broad, not much elongated, marked with several (commonly five) weak radial ribs without development of any prominent spiny scales; disk sculpture and other morphological characters essentially similar to those of C. mux nux.
Remarks. —When Oyama in Taki and Oyama (1954) made a taxonomic revision on Yokoyama's illustrated molluscs from the Quaternary of Kanto region, a new name, Aequipecten (Cryptopecten) sematensis, was introduced for two tumid specimens of a pectinid which had been referred to Pecten tissoti [error of tissotii] by Yokoyama (1922).
Two different views are possible as to the validity of Oyama's proposal of this taxonomic name. One interpretation, as pointed out by Hayami (1973) and Masuda and Noda (1976), is that A. (C.) sematensis Oyama, 1954, is a nomen nudum, because the proposal was not accompanied by any indication of diagnostic characters. Under this interpretation the present description would constitute the first valid proposal. The other view, as personally communicated by Dr. K. Oyama (October 16, 1982), is that the taxonomic name has been available since 1954, because the citation [synonym list first appeared in Oyama (1973)] of Yokoyama's description of "Pecten tissoti [sic]" constitutes and valid indication as required by ICZN articles 13 (a) (ii) and 16 (a) (i). I am now in favor of the latter interpretation, applying Oyama's trivial name, sematensis, for this taxon.
My examination of one of Yokoyama's specimens (UMUT CM21561) (the other specimen CM21562, which was erroneously designated as the holotype of A. (C.) sema tensis by Oyama (1973), is missing from the University Museum, the University of Tokyo), and also of a newly collected sample Sm (N), showed that the structure of radial ribs and many other characteristics are quite similar to those of C. nux from other fossil localities and the present seas. The convexity of the two valves is, however, unusually strong, and the thinkness (T) is far more positively allometric to length (L) and height (H). The difference in the shell convexity and growth ratio (a) between this subspecies and C. nux nux is clearly recognizable from the profiles of the two valves (Pl. 3, Fig. lc versus Fig. 3c; Fig. 2c versus Fig. 4c) as well as the discrepant slopes of reduced major axes in the double logarithmic diagrams of relative growth (see Figs. 15 and 16), Another criterion for the subspecific distinction seems to exist in the shape and sculpture of the byssal wing. In the present subspecies the byssal wing is not so narrowly elongated nor so strongly ornamented as in the nominate subspecies.
Distribution. —This subspecies has been known almost exclusively from the Yabu Formation at Ochishimoshinden of Semata, Chiba Prefecture. I have found only one left valve of this subspecies in the old collection of the Tohoku University (IGPS no. 13369), a specimen which was collected by Prof. Yabe from "Narita Beds at Takakura, Shito Village" and registered as Pecten (Chlamys) vesiculosus, Late Pleistocene (ca, 0.29 Ma).
Cryptopecten vesiculosus (Dunker)
Diagnosis. —Medium- or large-sized species of Cryptopecten characterized by relatively thick test, moderately strong convexity of both valves, tall outline, small apical angle and 13-18 radial ribs, each of which consists of a flat-topped central solid ridge and a pair of hollow parts covered by oppositely disposed imbricated scales.
Remarks. —C. vesiculosus is morphologically distinct from C. bullatus and C. nux in the larger shell size, much fewer radial ribs and oppositely disposed imbricated scales on the sides of each rib. The shell convexity is generally stronger than that of C. bullatus and weaker than that of C. nux. In accordance with the present proposal of a new subspecies for two Pliocene fossil samples from the Kakegawa Group of central Japan, all the Recent and other fossil samples of this species are assigned to its nominate subspecies, Cryptopecten vesiculosus vesiculosus.
Cryptopecten vesiculosus vesiculosus (Dunker)
Pl. 3, Fig. 5; Pl. 4, Figs. 1-3; Pl. 5, Figs. 1-3; Pl. 6, Figs. 1-6; Pl. 7, Figs. 1-10; Pl. 10, Figs. 1, 2; Pl. 11, Figs. 1, 2; Pl. 12, Fig. 12; Pl. 13, Figs. 1, 2
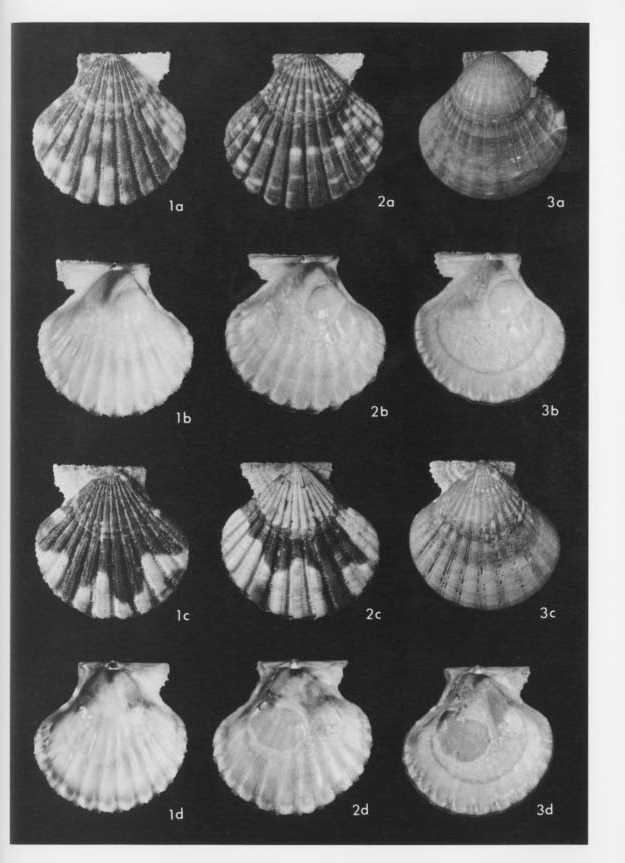 Explanation of Plate 4 (All figures ×1.5) Figs. 1-3. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 1. Living conjoined valves (UMUT RM16138b), Phenotype Q (rough subphcnotype), sample Is, mouth of Ise Bay; 1a: external view of right valve, 1b: internal view of right valve, 1c: external view of left valve, 1d: internal view of left valve. Fig. 2. Living conjoined valves (UMUT RM16138c), Phenotype Q (smooth subphenotype), the same sample; 2a: external view of right valve, 2b: internal view of right valve, 2c: external view of left valve, 2d: internal view of left valve. Fig. 3. Living conjoined valves (UMUT RM16138d), Phenotype R, the same sample; 3a: external view of right valve, 3b: internal view of right valve, 3c: external view of left valve, 3d: internal view of left valve. |
 Explanation of Plate 5 (× 1.5 for Figs. 1a-c, 2a, b, 3a-c, ×4.5 for Figs. 1d-f, 2c, d, 3d-f) Figs. 1-3. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 1. Living conjoined valves (UMUT RM16138b), the same specimen as Pl. 4, Fig. 1; 1a: anterior view, 1b: dorsal view, 1c: ventral view, 1d: byssal wing (with whitening), 1e: ventral area of right valve (with whitening), 1f: ventral area of left valve (with whitening). Fig. 2. Living conjoined valves (UMUT RM16138c), the same specimen as Pl. 4, Fig. 2; 2a: anterior view, 2b: dorsal view, 2c: ventral area of right valve (with whitening), 2d: ventral area of left valve (with whitening). Fig. 3. Living conjoined valves (UMUT RM16138d), the same specimen as Pl. 4, Fig. 3; 3a: anterior view, 3b: dorsal view, 3c: ventral view, 3d: byssal wing (with whitening), 3e: ventral area of right valve (with whitening), 3f: ventral area of left valve (with whitening). |
 Explanation of Plate 6 (All figures × 1.5) Figs. 1-6. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 1. Right valve (UMUT CM16051a), Phenotype Q (rough subphenotype), sample Ab 1, Late Pleistocene Jizôdô Formation at Atebi of Boso Peninsula; 1a: external view, 1b: dorsal view, 1c: anterior view. Fig. 2. Right valve (UMUT CM16051b), Phenotype Q (smooth subphenotype), the same sample; 2a: external view, 2b; dorsal view, 2c: anterior view. Fig. 3. Right valve (UMUT CM16051c), Phenotype R, the same sample; 3a: external view, 3b: dorsal view, 3c: anterior view. Fig. 4. Left valve (UMUT CM16051d), Phenotype Q (rough subphenotype), the same sample; 4a: external view, 4b: dorsal view, 4c: anterior view. Fig. 5. Left valve (UMUT CM16051e), Phenotype Q (smooth subphenotype), the same sample; 5a: external view, 5b: dorsal view, 5c: anterior view. Fig. 6. Left valve (UMUT CM16051f), Phenotype R, the same sample; 6a: external view, 6b: dorsal view, 6c: anterior view. |
 Explanation of Plate 7 (All figures × 1.5, unless otherwise stated) Figs. 1-10. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 1. Right valve (UMUT CM16057a), Phenotype Q (rough subphenotype), sample Ms, Holocene Moeshima Shell Bed at Moeshima Islet near Kagoshima. Fig. 2. Left valve (UMUT CM16057b), Phenotype Q (rough subphenotype), the same sample. Fig. 3, Right valve (UMUT CM16057c), Phenotype Q (smooth subphenotype), the same sample. Fig. 4. Left valve (UMUT CM16057d), Phenotype Q (smooth subphenotype), the same sample. Fig. 5. Right valve (UMUT CM16057e), Phenotype R, the same sample. Fig. 6. Left valve (UMUT CM16057f), Phenotype R, the same sample. Fig. 7. Right valve (UMUT CM16038a), Phenotype Q,, sample Tm 2, Middle Pleistocene Umegase Formation at Tsujimori of Boso Peninsula. Fig. 8. Left valve (UMUT CM16038b), Phenotype Q,, the same sample. Fig. 9. Conjoined valves (UMUT CM16030a), Phenotype Q., sample Ik, Late Pliocene Shinzato Formation at Ikei Islet, Okinawa, 9a: external view of right valve, 9b: external view of left valve, 9c: anterior view, 9d: dorsal view. Fig. 10. Conjoined valves (UMUT CM16030b), Phenotype Q,, the same sample; external view of right valve. Figs. 11,12. Cryptopecten vesiculosus makiyamaisubsp. nov. Fig. 11. Right valve (UMUT CM16033b), Paratype, sample Kg 1, Late Pliocene Hosoya Silt of Kakegawa Group at west of Ugari near Fukuroi.(×1) Fig. 12. Left valve (UMUT CM16033a), Holotype, the same sample ( × 1) |
 Explanation of Plate 13 (SEM photographs, all figures × 29, 5 KV) Figs. 1, 2. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 1. Living conjoined valves (UMUT RM16138a), Phenotype Q,, sample Is, mouth of Ise Bay; 1a: transverse section of radial rib at middle part of disk of right valve; 1b: oblique view of interspace of radial ribs at ventral margin of right valve, 1c: transverse section of radial rib at middle part of disk of left valve; 1d: oblique view of interspace of radial ribs at ventral margin of left valve. Fig. 2. Living conjoined valves (UMUT RM16061a), Phenotype R, sample Jg (2), off Jôgashima Islet, Sagami Bay; 2a: transverse section of radial rib at middle part of disk of right valve, 2b: oblique view of interspace of radial ribs at ventral margin of right valve, 2c: transverse section of radial rib at middle part of disk of left valve, 2d: oblique view of interspace of radial ribs at ventral margin of left valve. |






1877. Pecten vesiculosus Dunker, Malakol. Bl., vol. 24, p. 72.
?1877. Pecten trifidus Dunker, Malakol. Bl., vol. 24, p. 72.
1882. Pecten vesiculosus Dunker: Dunker, Index Molluscorum rnaris Japonici, p. 241, pl. 11, fig. 1 [Phenotype Q].
?1882. Pecten jickelii Dunker, Index Molluscorum maris Japonici, p. 241. [new name for Pecten trifidus Dunker, 1877].
1888. Pecten vesiculosus Dunker: Küster and Kobelt in Martini and Chemnitz, System. Conch. Cab., vol. 7, p. 138, pl. 38, fig. 4 [Phenotype Q].
?1888. Pecten hysginodes Melvill, Jour. Conch., vol. 5, p. 280, pl. 2, fig. 8 [? Phenotype R].
1902. Pecten vesiculosus Dunker: Yoshihara, Zool. Mag. Tokyo, vol. 14, p. 212, pl. 4, fig. 16 [Phenotype Q].
1911. Pecten vesiculosus Dunker: Yokoyama, Jour. Geol, Soc, Tokyo, vol. 18, p. 1, pl. 1, figs. 8-10 [Phenotype Q].
1920. Pecten vesiculosus Dunker: Yokoyama, Jour. Coll. Sci. Imp. Univ. Tokyo, vol. 39, art. 6, p. 154, pl. 13, figs. 11-13 [Phenotype Q}.
1922, Pecten vesiculosus Dunker: Yokoyama, Jour. Coll. Sci. Imp. Univ. Tokyo, vol. 44, art. 1, p.182.
1925. Pecten vesiculosus Dunker: Yokoyama, jour. Coll. Sci. .Imp. Univ. Tokyo, vol. 45, art. 5, p. 27.
1928. Pecten vesiculosus Dunker: Yokoyama, Jour. Fac. Sci. Imp. Univ. Tokyo, sec. 2, vol. 2, pt. 7, p.349.
1929. Chlamys (Aequipecten) vesiculosus [sic] (Dunker): Fujita, Venus, vol. 1, no. 1, p. 60.
1932. Chlamys (Aequipecten) vesiculosus [sic] (Dunker): Kuroda, Venus, vol. 3, no. 2, p. 94(app.), fig. 106 [Phenotype Q], fig. 107 [Phenotype R].
1934. Chlamys (Aequipecten) vesiculosa (Dunker): Hirase, Collection of Japanese Shells, p. 8, 111, pl. 12, fig. 6 [Phenotype Q].
1951. Cryptopecten vesiculosus (Dunker): Habe, Genera of Japanese Shells, p. 77, figs. 155-158.
1951. Cryptopecten vesiculosus (Dunker): Taki in Hirase, Illustrated Handbook of Shells, pl. 12, fig. 6 [Phenotype Q].
1954. Aequipecten (Cryptopecten) vesiculosus (Dunker); Taki and Oyama, Palaeont. Soc, Japan, Spec. Papers, no. 2, pl. 14, figs, 11-13 [Phenotype Q] [reproduction of Yokoyama's (1920) figures].
1954. Cryptopecten vesiculosus (Dunker): Kira, Coloured Illustrations of the Shells of Japan, p. 123, pl. 49, fig. 1 [Phenotype Q].
1958. Cryptopecten vesiculosus (Dunker): Habe, Publ, Seto Mar. Biol. Lab., vol. 6, no, 3, p. 265.
1958. Chlamys vesiculosa (Dunker): Shirai, Venus, vol. 20, no. 1, p. 88.
1960. Cryptopecten vesiculosus (Dunker): Shuto, Mem. Fac. Sci. Kyushu Univ., ser. D, vol. 9, no. 3, p. 123, pl. 13, fig. 3 [Phenotype Q].
1962. Aequipecten vesiculosus (Dunker): Masuda, Sci. Rep. Tohoku Univ., ser. 2, vol. 33, no. 2, p. 191, pl. 24, figs. 7, 8, pl. 25, fig. 7 [Phenotype Q].
1967. Cryptopecten vesiculosus (Dunker): Habe and Kosuge, Shells, p. 134, pl. 50, fig. 12 [Pheno type R], fig. 13 [Phenotype Q].
1968. Aequipecten vesiculosus (Dunker): Ohara, Geol. Atlas Chiba Pref., no. 5. Bivalvia, pl. 3, fig. 6a, b [Phenotype Q].
1969. Aequipecten (Cryptopecten) vesiculosus (Dunker): Shikama and Masujima, Sci. Rep. Yoko hama Nat. Univ., ser. 2, no. 15, pl. 7, fig. 14 [Phenotype Q].
1971. Cryptopecten vesiculosus (Dunker): Kuroda, Habe and Oyama in Biological Laboratory, Imperial Household, Seashells of Sagami Bay, p. 574/366, pl. 79, figs. 1-4 [Phenotype Q], figs. 5-7 [Phenotype R].
1972. Cryptopecten vesiculosus (Dunker); Hayami, Jour. Geol. Soc. Japan, vol. 78, no. 9, p. 495, 499, text-fig. 1 (left) [Phenotype Q], text-fig. 1 (right) [Pehnotype R].
1973. Cryptopecten vesiculosus (Dunker): Hayami, jour. Paleontology, vol. 47, no. 3, p. 401, pl. 1, figs. 1-4, 9,10, pl. 2, figs. 1, 2, 7, 8 [Phenotype R], pl. l, figs. 5-8,11, 12, pl. 2, figs. 3-6, 9-12 [Phenotype Q].
1973. Aequipecten vesiculosus (Dunker): Hayasaka, Sci. Rep. Tohoku Univ. ser. 2, spec. vol., no. 6, p. 102, pl. 6, fig. 3 [Phenotype Q].
1973. Aequipecten (Cryptopecten) vesiculosus (Dunker): Oyama, Palaeont. Soc. Japan, Spec. Papers, no. 17, p. 85, pl. 34, figs. 1-3 [Phenotype Q] [reproduction of Yokoyama's (1920) figures].
1975. Cryptopecten vesiculosus (Dunker): Okutani and Habe, Mollusca II chiefly from Japan, p. 90, 4 figs. [Phenotype R], p. 255.
1975. Cryptopecten vesiculosus (Dunker): Hayami and Ozawa, Lethaia, vol. 8, p. 10, fig. 9 (left) [Phenotype R], fig. 9 (right) [Phenotype Q].
1977. Cryptopecten vesiculosus (Dunker): Habe, Systematics of Mollusca in Japan, p. 84, pl. 16, figs. 3-5.
1979. Cryptopecten vesiculosus (Dunker): Nemoto and Ohara, Taira Chigaku Dokokai Kaiho, spec. vol., p. 56, pl. 3, fig. 3 [Phenotype Q].
1979. Aequipecten (Cryptopecten) vesiculosus (Dunker): Mori and Osada, Mater. Rep. Hiratsuka City Museum, no. 19, p. 38, pl. 8, fig. 10 [Phenotype Q].
1979. Aequipecten (Cryptopecten) sematensis Oyama: Mori and Osada, Mater. Rep. Hiratsuka City Museum, no. 19, p. 38, pl. 8, fig. 11 [Phenotype R] [non Aequipecten (Cryptopecten) sematensis Oyama, 1954].
1982. Cryptopecten vesiculosus (Dunker); Hayami, Venus, vol. 41, p. 235.
Type. —The original description of Pecten vesiculosus by Dunker (1877) was not accompanied by any illustrations, but a specimen subsequently figured by the same author (Dunker, 1882, pl. 11, fig. 1) is presumably one of the syntypes. The locality mentioned was Japan, but details are unknown.
Material. —Numerous specimens, as indicated in the list of examined samples (p. 127), were used for the present description and discussions.
Diagnosis. —Medium-sized subspecies of Cryptopecten vesiculosus characterized by relatively small number of radial ribs, thick test with well-developed inner shell layer and broad central solid ridge of radial rib. Middle Pleistocene and later populations remarkably dimorphic in surface sculpture. This subspecies occurs predominantly on coarse-grained substrates and in sandy sediments.
Description. —Shell rarely exceeding 34 mm (38 mm in fossils) in length and height, highly inequivalve, inequilateral, nearly acline throughout growth, left-convex in young stages but becoming right-convex in adult; height always negatively allometric to length, commonly surpassing length in young but subequal to or rather smaller than length in adult; thickness positively allometric to length (and height) in right valve but commonly negatively allometric in left; form ratio T/L as large as 0.25-0.30 in adult right valve and 0.20-0.24 in adult left valve; length of hinge line moderate, negatively allometric to overall length; test comparatively thick; apical angle of disk 90-95 degrees in Recent specimens but often exceeding 95 degrees in Pliocene fossils; anterior wing about twice as long as posterior; byssal wing comparatively broad, with elevated dorsal margin above hinge axis; posterodorsal margin of disk slightly concave and slightly longer than antero dorsal in adult, but subequal in length in young; byssal notch moderately deep, with three or four exposed denticles of ctenolium and relatively narrow fasciole area; antero dorsal and posterodorsal margins of disk scarcely gaped between two valves.
Outer surface of disk ornamented with 13-18 broad, regular, radial ribs; structure of radial sculpture quite different in two phenotypes, as separately described; very conspicuous stepwise growth ring(s) frequently occur in adult individuals; byssal wing commonly ornamented with five (sometimes four or six) strong radial ribs, on which a number of finny scales occur; other wings also ornamented with several radial riblets, upper one or two of which are commonly stronger than the rest.
Coloration variable, but commonly reddish brown in ground color, often variegated with pale bands and spots; contrast between darkly pigmented part and pale part more remarkable in left valve than in right; byssal wing and early dissoconch of both valves almost invariably pale in color.
Inner surface porcellaneous in luster except for vitreous muscle portion owing to well developed inner foliate layer, almost white in right valve but more or less darkly pigmented in left valve; radial ribs weakly impressed on central-ventral area; adductor muscle scar clearly impressed, moderate in size, consisting of striate (quick) muscle portion and smooth (slow) muscle portion, which, however, are obscurely discriminated in left valve; position of striate muscle scar somewhat different between two valves owing to oblique direction of quick muscle; resilial insertion moderate in size, bordered by a pair of strong cardial crura.
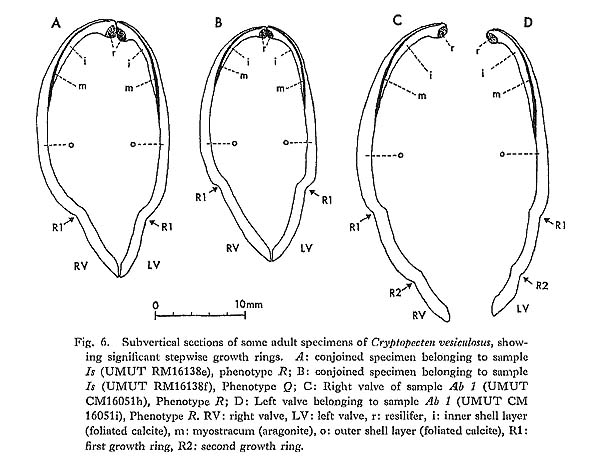
Sculpture difference in two phenotypes. —The radial ribs on the disk are highly elevated and subquadrate in transverse section in Phenotype Q, whereas they are rounded and very low in Phenotype R. The central solid ridge of each radial rib is flat-topped and comparatively wide in both phenotypes. Its surface is always smooth in Phenotype R, but may or may not have fine erect scales in Phenotype Q. In both phenotypes the central ridge is broader in the right valve than in the left. In Phenotype Q, however, the sculpture is essentially the same in both valves, the lateral slopes of the solid ridge being steep and forming a pair of hollow parts which are covered by imbricated scales numbering about three per 1 mm on the middle-ventral surface of adult shell. In the same phenotype the interspace between ribs possesses fine erect scales which are never imbricated and more distantly spaced than the scales on the hollow parts. On the other hand, in Phenotype R the radial sculpture is considerably different in the two valves; in the right valve the inter- space is quite narrow in the young stage and not clearly discriminated from the slopes of ribs in the adult, while in the left valve it is wide and completely covered by imbricated scales numbering about eight per 1 mm on the middle-ventral surface of adult shell, which are quite analogous with those on the hollow parts, not only in shape but also in periodicity. In other words, hollow parts are restricted to the lateral sides of the central ridge in Phenotype Q, whereas imbricated scales also cover the entire rib interspace of the left valve in Phenotype R. In the right valve, the hollow parts are persistent in Phenotype Q but have completely vanished on the ventral surface in Phenotype R. In transverse section, the hollow space is lenticular in Phenotype Q but subcircular in Phenotype R. In both phenotypes the hollow space is always wider in the left valve than in the right. Radial ribs on the wings are invariably solid without any hollow space, and their structure is essentially the same in the two phenotypes.
Remarks. —Cryptopecten vesiculosus is a common pectinid in Japan, but its morphology and variation have not been studied in detail. The remarkable discontinuous variation of disk sculpture, however, seems to have been noticed by some previous authors. For example, Kuroda (1932) illustrated two left valves belonging to different phenotypes in juxtaposition. Furthermore, Habe and Kosuge (1967) and Kuroda, Habe and Oyama in Biological Laboratory, Imperial Household (1971) mentioned, though briefly, the presence of dimorphic phenomenon in their materials. Fortunately, however, except for Kira (1950) and Mori and Osada (1979), no one seems to have regarded the two pheno types as separable into different taxa. As already documented in this paper, the two phenotypes coexist in every large sample after the Middle Pleistocene and are surely attributable to intrapopulational discontinuous variation.
Some problems about the synonymy of early proposed specific names remain unsolved. Pecten trifidus Dunker, 1877, from Japan, which was renamed Pecten jickelii by Dunker (1882) without any adequate reason, may be conspecific with P. vesiculosus, as was suggested by Habe (1977). The original descriptions of P. trifidus and P. jickelii were not accompanied by any illustrations, but the number of radial ribs, which was said to be 18 in P. trifidus, may be somewhat larger than the averages in the present samples of C. vesiculosus. Their taxonomic position, therefore, is difficult to determine without examining the type material. On the basis of my observation of Dr. T. R. Waller's unpublished photographs, I conclude that the type conjoined specimen of Pecten hysginodes Melvill, 1888, from unknown locality, which is preserved in the Cardiff Museum, may belong to the Phenotype R of C. vesiculosus.
The detailed morphology and phenotypic frequency are, as described in the preceding chapters, considerably variable in time and space, but all the samples here treated certainly belong to a single evolutionary species. Masuda (1962) mentioned the existence of some morphological difference between the Pliocene and Pleistocene-Recent specimens of this species. He pointed out that some intercostal threads are developed in the Recent and Pleistocene specimens near the ventral margin, but that they are usually absent in Tertiary ones. Furthermore, the Pliocene specimens were said to have broader ribs and narrower interspaces than Pleistocene-Recent ones. My observation of the present samples of various ages revealed these characters to be more closely related to phenotypic and subphenotypic differences, ontogenetical change and state of preservation than to chronological change. The intercostal sculpture actually differs not only between the two phenotypes but also between the two subphenotypes (p. 58) within Phenotype Q; inter costal threads commonly appear in adult individuals of rough subphenotype but are rarely met with in those of smooth subphenotype almost regardless of geological horizons. Many well-preserved large specimens from the Pliocene, for example, those in samples Sh 1 and Ik, show as clear intercostal threads as those of Pleistocene-Recent samples, while smaller specimens always lack such threads. The relative breadth of radial ribs varies considerably among individuals within one sample and increases remarkably with growth. A striking feature is that radial ribs appear very narrow if imbricated scales on their sides are exfoliated. Though biometrical comparison of this character among samples has not been carried out owing to the different growth stages and difficulty of subphenotypic discrimination in many unfavorably preserved samples, chronological change could not be detected with respect to the relative breadth of ribs and interspaces.
Distribution. —This subspecies lives on the outer sublittoral sandy substrates around the central and southwestern parts of the Japanese Islands under the influence of Kuroshio warm current (35°N and south) and its branch, Tsushima Current (41°N and south) (see Figs. 1 and 3). Fossils of this subspecies are also almost entirely restricted to the same area as the distribution in the present time, though the northern limit seems to have fluctuated with time (see Fig. 4). Middle Pliocene (ca. 3.5 Ma) to Recent.
Cryptopecten vesiculosus makiyamai subsp. nov.
Pl. 7, Figs. 11, 12
 Explanation of Plate 7 (All figures × 1.5, unless otherwise stated) Figs. 1-10. Cryptopecten vesiculosus vesiculosus (Dunker) Fig. 1. Right valve (UMUT CM16057a), Phenotype Q (rough subphenotype), sample Ms, Holocene Moeshima Shell Bed at Moeshima Islet near Kagoshima. Fig. 2. Left valve (UMUT CM16057b), Phenotype Q (rough subphenotype), the same sample. Fig. 3, Right valve (UMUT CM16057c), Phenotype Q (smooth subphenotype), the same sample. Fig. 4. Left valve (UMUT CM16057d), Phenotype Q (smooth subphenotype), the same sample. Fig. 5. Right valve (UMUT CM16057e), Phenotype R, the same sample. Fig. 6. Left valve (UMUT CM16057f), Phenotype R, the same sample. Fig. 7. Right valve (UMUT CM16038a), Phenotype Q,, sample Tm 2, Middle Pleistocene Umegase Formation at Tsujimori of Boso Peninsula. Fig. 8. Left valve (UMUT CM16038b), Phenotype Q,, the same sample. Fig. 9. Conjoined valves (UMUT CM16030a), Phenotype Q., sample Ik, Late Pliocene Shinzato Formation at Ikei Islet, Okinawa, 9a: external view of right valve, 9b: external view of left valve, 9c: anterior view, 9d: dorsal view. Fig. 10. Conjoined valves (UMUT CM16030b), Phenotype Q,, the same sample; external view of right valve. Figs. 11,12. Cryptopecten vesiculosus makiyamaisubsp. nov. Fig. 11. Right valve (UMUT CM16033b), Paratype, sample Kg 1, Late Pliocene Hosoya Silt of Kakegawa Group at west of Ugari near Fukuroi.(×1) Fig. 12. Left valve (UMUT CM16033a), Holotype, the same sample ( × 1) |


Type. —The holotype is a left valve (UMUT CM 16033a) from the Hosoya Silt of the Kakegawa Group at the west of Ugari, northeast of Yamanashi, Fukuroi City, Shizuoka Prefecture, central Honshu (34°47.5'N, 137°56.3'E). 44.5 mm long, 43.1 mm high, 9.9 mm thick.
Material (Paratypes). —In addition to the holotype 25 specimens of the samples Kg 1 from the type locality and Kg 2 from a nearby locality are concerned with the following description and discussions. See also the list of examined samples.
Diagnosis. —Unusually large-sized subspecies of Cryptopecten vesiculosus characterized by rapid growth rate, relatively large number of radial ribs, thin test and narrow central solid ridge of radial rib. This subspecies characteristically occurs in silty sediments.
Discription. —Shell often exceeding 40 mm in length and height; test comparatively thin; apical angle approximately 95 degrees; 15-18 radial ribs, with an average of 16, each of which consists of a comparatively narrow central ridge and a pair of relatively wide hollow parts; rib interspace marked with a number of fine radial threads in adult shell, showing lattice ornament with fine erect scales; other characteristics essentially similar to those of Phenotype Q of C. vesiculosus vesiculosus.
Remarks. —Since Makiyama's (1931) stratigraphic work, it has been known that C. vesiculosus occurs frequently in the Hosoya Silt of the Kakegawa Group in Ugari area of Fukuroi City. No paleontological description, however, has been made on the material of this member.
Owing to the small sample size and appreciable secondary deformation, detailed morphometric comparison with other samples is still difficult. The specimens from the Hosoya Silt (samples Kg 1 and Kg 2) are, however, morphologically and paleoecologically very unique in the following points. Firstly, the maximum shell size and growth rate are unusually large as shown in Table 13 and Fig. 7. In spite of the large size, the test is comparatively thin. Secondly, the central solid ridge of each rib is much narrower, and the hollow parts, though the covering imbricated scales have been lost in every specimen, are somewhat wider than in other samples of C. vesiculosus.Thirdly, the average number of radial ribs on the disk is slightly but significantly larger than that in any other sample of this species, as indicated in Table 11. Fourthly, as discussed already (p. 80), the habitat and associated molluscs seem to be considerably different from those of Recent and other fossil populations of C. vesiculosus. This subspecies is found exclusively in silty beds. Because the age of the Hosoya Silt is certainly within the range of C. vesiculosus, I provesionally regard this phenon as constituting a geographical (or ecological) subspecies.
Distribution. —This subspecies is known only from the Hosoya Silt Member of the Kakegawa Group in central Honshu, Japan. Late Pliocene (1.9-2.4 Ma).
Cryptopecten phrygium (Dall)
PI. 9, Figs. 6-9
1886. Pecten phrygium Dall, Bull. Mus. Comp. Zool., Harvard Coll., vol. 12, no. 6, p. 217, pl. 40, fig.l.
1954. Aequipecten (Aequipecten) phrygium Dall: Abbott, American Seashells, p. 367.
1974. Aequipecten phrygium (Dall): Abbott, American Seashells, 2nd ed., p. 445.
1982. Cryptopecten phrygium (Dall): Woodring, U. S. Geol. Surv. Prof. Paper, 306-F, p. 595.
Type. —The illustrated syntype is a living conjoined specimen dredged at Station 32 of the U.S. Coast Survey Steamer Blake in 95 fathoms, north of Yucatan Banks (23°32'N, 88°05'W) in the Mexican Gulf. 36.5 mm long, 36.5 mm high. Several dead shells, which also constitute the syntypes, were collected at three other stations in the same gulf. These specimens are preserved in the Museum of Comparative Zoology, Harvard University.
Material. —Several small samples in the National Museum of Natural History, Washington D. C., and the American Museum of Natural History, New York, are concerned with the following description and discussions, as indicated in the list of examined samples.
Diagnosis. —Large-sized species of Cryptopecten characterized by weak convexity of both valves, relatively short hinge line, and 17-19 radial ribs with sharply angulated central solid ridge and regularly alternate imbricated scales.
Description. —Shell sometimes exceeding 45 mm in length and 43 mm in height, inequivalve, inequilateral, nearly acline in young stages but considerably prosocline in adult, relatively tall in young but rather long in adult, equiconvex or slightly right-convex; test moderate in thickness; hinge line decidedly short in adult stages, though rather long in young; posterodorsal margin of disk broadly concave, much longer than anterodorsal; apical angle of disk about 100 degrees; wings relatively small in adult stages; byssal notch moderately deep, provided with about five exposed denticles of ctenolium and narrow fascicle area.
Outer surface of disk ornamented with 17-19 highly elevated radial ribs, each of which is reinforced by an angular solid ridge; hollow parts on both sides of ridges covered with alternately disposed imbricated scales, which number about five per 1 mm on middle-ventral surface of adult shell; rib interspace moderate in width, marked with fine scales and in adult stage, with a few fine intercostal threads as well; both wings orna- mented with several weak radial riblets; growth rings, if present, rather inconspicuous.
Coloration considerably variable, as in other species of this genus, and commonly mottled with reddish ground and irregular pale spots.
Inner surface porcellaneous in luster owing to well-developed inner foliated layer, almost white in right valve but more or less darkly pigmented in left valve; radial ribs impressed on central-ventral area; muscle impression similar to that of C. vesiculosus; cardinal crura comparatively weak.
Remarks. —Cryptopecten phrygium is a solitary representative of this genus in the Atlantic. It may be an uncommon species, judging from. the scarceness of subsequently described specimens. In the National Museum of Natural History and the American Museum of Natural History, there are many small samples which agree well with the original description, but morphometrical treatment of the intrapopulational and geo- graphic variations and allometric growth is difficult owing to the small number of individuals. Nevertheless, a highly negatively allometric growth of H to L as well as of D to L is strongly suggested. In short, every essential character indicates that this species is closely related to Pacific species of Cryptopecten.
It resembles C. bullatus in the weak convexity of both valves and the mode of radial sculpture characterized by the sharp central ridge and regularly alternate imbricated scales on the hollow parts. However, the maximum shell size is almost twice as large as that of C. biillatus. If specimens of similar size from the two species are compared, it is easy to see that C. phrygium has much taller shell than C. bullatus. Moreover, the average number of radial ribs is decidedly fewer in the present species. In the developed inner layer and clearly impressed muscle scars it may be rather similar to C. vesiculosus, but clearly differs from the Japanese species in the more numerous radial ribs, narrower central ridge, weaker shell convexity and alternate disposition of imbricated scales.
Distribution. —Living specimens of this species are known from a depth of about 200 meters. In. addition to many stations in the Gulf of Mexico, this species has been known from the Atlantic coast (from Cape Cod to Florida), the Lesser Antilles and a station off Georgetown in Guiana. Recent.
Cryptopecten spinosus sp. nov.
Pl. 8, Figs. 1-5; Pl. 12, Figs. 1,2
1934. Pecten (Aequipecten) vesiculosus Dunker: Nomura and Zinbo, Sci. Rep. Tohoku Imp. Univ., scr. 2, vol. 16, no. 2, p. 117. [non Pecten vesiculosus Dunker, 1877]
Type. —The holotype is a nearly complete right valve (UMUT CM16170c) from the coral sand of the Late Pleistocene Wan Formation of the Ryukyu Group at about 500 m north of Kamikatetsu, Kikai Island (Kikai Town), Kagoshima Prefecture, south Japan (28°17.0'N, 129°56.8'E). 25.7 mm long, 25.1 mm high, 6.1 mm thick.
Material (Paratypes). —In addition to the holotype, 189 specimens of the sample Kk (S) from the type locality are concerned with the following description and discussions.
Diagnosis. —Medium-sized species of Cryptopecten, characterized by relatively weak convexity of left valve, 12-15 radial ribs, a narrow and sharp central ridge which is spinously projected beyong ventral margin, somewhat coarse and oppositely disposed imbricated scales covering tubular hollow parts, and conspicuous scales on byssal wing.
Description. —Shell rarely exceeding 26 mm in length and height, highly inequivalve, subequilateral, nearly acline throughout growth, right-convex except for very early stages of dissoconch; height commonly exceeding length in young stages, but negatively allometric and becoming subequal to length in adult stages; thickness positively allo metric to length in right valve but isometric or rather negatively allometric in left valve; form ratio T/L as small as 0.23-0.27 in adult right valve and 0.17-0.19 in adult left valve; hinge line comparatively short, negatively allometric to overall length; test solid, moderate in thickness; wings highly unequal in size; pre-umbonal dorsal margin of right valve elevated above hinge axis; posterodorsal margin of disk slightly concave, a little longer than anterodorsal; apical angle about 90-95 degrees; byssal notch considerably deep, provided with three or four exposed denticles of ctenolium and relatively narrow fasciole area.
Outer surface of disk ornamented with 12-15 highly elevated and strong radial ribs; each rib reinforced by a narrow, flat-topped and scaly central solid ridge, the sides of which are steep and remarkably excavated; imbricated scales oppositely disposed, coarse (about four per 1 mm), strongly convex and granular in appearance; consequently, hollow space covered with imbricated scales comparatively wide and tubular; terminal of each central ridge spinously projected beyond ventral margin and observable also from inside of shell; rib interspace moderate in width, marked with erect scales which are quite in dependent from and more distantly spaced than imbricated scales on hollow parts; fine intercostal threads occurring only in adult stages; byssal wing ornamented with four (occasionally five) radial riblets on which several finny scales are developed; other wings marked with weaker riblets; stepwise growth rings sometimes observed on adult shell.
Color pattern recognizable, though obscurely, on external and internal surface especially in left valve, characterized by irregular bands and spots on darkly pigmented ground. Internal surface covered with thin inner shell layer with clearly impressed radial sculpture except for umbonal area; cardinal crura relatively weak; muscle scars not much different from those of C. vesiculosus vesiculosus in size and shape.
Remarks. —The present species, as interpreted by Nomura and Zinbo (1934), resembles C. vesiculosus, but is clearly distinguishable from that species by the weaker convexity of both valves (see Figs. 15 and 16), significantly fewer radial ribs (Table 10) and central ridge projected beyond ventral margin. One of the most distinctive characters is the shape of the imbricated scales on the hollow parts of the ribs. In Phenotype Q of C. vesiculosus the slopes of the central solid ridge are never so deeply excavated, and the imbricated scales are not so strongly convex. Consequently, in the transverse section and also in ventral view, the hollow space is lenticular in Phenotype Q of C. vesiculosus but nearly circular in the present species. In short, the sculpture of C. spinosus is more prominent and decorative than that of C. vesiculosus and other species of Cryptopecten.
The present new species is known only from raised reef sediments of the Late Pleistocene age, and appears to have soon become extinct. Its reproductive isolation from C. vesiculosus before this stage is suggested by the absence of dimorphism in the sample Kk(S). I presume that C. spinosus represents an incipient species which was derived from the main stock of C. vesiculosus.
Distribution. —The present species is known only from the type locality. According to Omura (1983), this fossil bed represents grainstone facies deposited at a depth of 40-100 meters, and its 230Th/234U age is 82,000±2,000 years B. P. as determined from three species of ahermatypic corals. Late Pleistocene.
Cryptopecten yanagawaensis (Nomura and Zinbo)
Pl. 8, Figs. 6-9
 Explanation of Plate 8 (All figures × 1.5, unless otherwise stated) Figs. 1-5. Cryptopecten spinosus sp. nov. Fig. 1. Right valve (UMUT CM16170d), Paratype, sample Kk (S), Late Pleistocene Wan Formation at Kamikatetsu, Kikai Island; 1a: external view, 1b: internal view. Fig. 2. Left valve (UMUT CM16170c), Paratype, the same sample; 2a: external view, 2b: internal view, 2c: dorsal view, 2d: anterior view. Fig. 3. Right valve (UMUT CM16170e), Holotype, the same sample; 3a: external view, 3b: internal view, 3c: dorsal view, 3d: anterior view, 3e: byssal wing. ( × 4) Fig. 4. Left valve (UMUT CM16170f), Paratype, the same sample; 4a: external view, 4b: internal view. Fig, 5. Ventral ar ea of right valve (UMUT CM16170g), Paratype, the same sample. ( × 4) Fig. 6-9. Cryptopecten yanagawaensis (Nomura and Zinbo) Fig. 6. Right valve (UMUT CM16171a), sample Mn (Y), Middle Miocene Moniwa Formation at Junishin near Natori. Fig. 7. Left valve (UMUT CM16171b), the same sample. Fig. 8. Left valve (UMUT CM16171c), the same sample. Fig. 9. Right valve (UMUT CM16171d), the same sample. |




1936. Pecten (Aequipecien?) yanagawaensis Nomura and Zinbo, Saito Ho-on Kai Mus. Res. Bull., no. 10, p. 337, pl. 20, fig. 2a, b.
1940. Pecten (Aequipecten) yanagawaensis Nomura and Zinbo: Nomura, Sci. Rep. Tohoku Imp.
Univ., ser. 2, vol. 21, no. 1, p. 19, pl. 1, figs. 10-13.
1958. Cryptopecten yanagawaensis (Nomura and Zinbo): Masuda, Trans. Proc. Palaeont. Soc.
Japan, n. s., no. 30, p. 189, pl. 27b, figs. 1-8.
1962. Aequipecten yanagawaensis (Nomura and Zinbo): Masuda, Sci. Rep. Tohoku Univ., ser. 2, vol. 33, no. 2, p. 192, pl. 26, fig. 8.
1965. Aequipecten yanagawaensis (Nomura and Zinbo): Masuda and Takegawa, Saito Ho-on Kai Mus. Res. Bull., no. 40, pl. 1, figs. 12, 13.
?1973. Aequipecten (Cryptopecten) yanagawaensis (Nomura and Zinbo): Shikama, Sci. Rep.
Tohoku Univ., ser. 2, spec. vol., no. 6, p. 190, 194.
1973. Aequipecten yanagawaensis (Nomura and Zinbo): Masuda, Atlas of Japanese Fossils, no. 33, pl. N-54, figs. 14-16.
1974. Cryptopecten yanagawaensis (Nomura and Zinbo): Itoigawa, Shibata and Nishimoto, Bull.
Mizunami Fossil Mus., no. 1, p. 67, pl. 11, figs. 6-9.
1976. Aequipecten yanagawaensis (Nomura and Zinbo): Ogasawara, Sci. Rep. Tohoku Univ., ser. 2, vol. 46, no. 2, p. 44, pl. 3, figs. 3, 6.
1981. Aequipecten yanagawaensis (Nomura and Zinbo): Itoigawa, Shibata, Nishimoto and Okumura, Monogr. Misunami Fossil Mus., no. 3A, pl. 7, figs. 2, 3.
1982. Aequipecten yanagawaensis (Nomura and Zinbo): Itoigawa, Shibata, Nishimoto and Okumura, Monogr. Mizunami Fossil Mus., no. 3B, p. 46.
1982. Cryptopecten yanagawaensis (Nomura and Zinbo): Hayami, Venus, vol. 41, no. 3, p. 235.
Type. —The, holotype in Saito Ho-on Kai Museum, Sendai (SM 8353) was collected from the early Middle Miocene Yanagawa Formation at a river cliff of the Hirose-gawa, the southwest end of Yanagawa Park, Yanagawa Town, Fukushima Prefecture, north Japan (37°51.1'N, 140°36.1'E). 21.5 mm long, 20 mm high, 5 mm thick.
Material. —The following description is based on the sample Mn (Y) from the early Middle Miocene Moniwa Formation of the Natori Group at the southwest of Junishin of Natori City, Miyagi Prefecture, north Japan. The type and other material of this species in the Saito Ho-on Kai Museum and Tohoku University were also examined.
Diagnosis. —Medium-sized species of Cryptopecten characterized by relatively weak convexity of both valves, low outline, large apical angle, 20-25 radial ribs with broad and flat-topped central ridge, oppositely disposed imbricated scales on ribs, strong cardinal crura and scarcely impressed radial ribs on internal surface, except for ventral periphery.
Description. —Shell barely exceeding 32 mm in length and height, nearly equiconvex or only slightly right-convex in adult stages, nearly acline in young stages but tending to become slightly prosocline in adult; height subequal to length in young but becoming somewhat smaller than length in adult; thickness appearing almost isometric to length (and height) throughout growth; form ratio T/L scarcely exceeding 0.27 in both valves; hinge line moderate in length and apparently negatively allometric to overall length; test rather thick; apical angle of disk about 95 degrees; wings moderate in size, anterior one slightly larger than posterior; anterodorsal and posterodorsal margins of disk slightly concave, subequal in length in young, but with the former becoming slightly longer than the latter in adult; byssal notch moderate in depth, with relatively narrow fasciole area; ctenolium commonly indistinct, something, however, which may be due to unfavorable preservation of examined material; pre-umbonal margin of right valve highly elevated above hinge axis.
Outer surface of disk ornamented with 20-25 radial ribs, each of which is reinforced by a broad and flat-topped central solid ridge and bordered by a pair of narrow lateral sulci originally covered by oppositely disposed imbricated scales; interspaces as broad as ribs, marked with densely spaced erect scales, and lacking intercostal threads even in adult shell; sculpture of disk in both valves essentially similar; stepwise growth ring(s) sometimes observed on disk; both wings of the two valves having several (five or so) radial riblets; scales apparently undeveloped on byssal wing.
Inner surface smooth, covered by thickly deposited inner shell layer, except for ventral marginal area; cardinal crura well developed especially in right valve.
Remarks. —The present species was fully described by Masuda (1958) on the basis of abundant specimens from the Moniwa Formation in the environs of Sendai. The present sample Mn (Y) coincides well with them and also with the holotype from the Yanagawa Formation. Masuda (1958) understandably regarded this species as an early repre sentative of Cryptopecten, though subsequently it was referred to Aequipecten together with some living species from Japan (Masuda, 1962; Masuda and Noda, 1976). Masuda (1958, 1962) noted that the number of radial ribs varies from 16 to 26 in his material, but my observation of the sample Mn (Y) and the pooled specimens in Tohoku University and the Saito Ho-on Kai Museum leads me to believe that the variation may not be so wide; most specimens possess 21 to 24 ribs. This conclusion seems to agree well with Mr. Y. Sato's unpublished morphometric data on some samples of the same species.
Owing to the coarse-grained matrix and pre-depositional abrasion, the detailed struc ture of the radial ornaments is in most cases difficult to observe, and imbricated scales on the hollow parts are almost entirely lost in most specimens. However, they are clearly observable in a few well-preserved specimens. The left valve illustrated by Masuda (1958, pl. 26b, fig. 8) is an exceptionally good specimen, showing the characteristic sculpture of Cryptopecten type and oppositely disposed imbricated scales.
Shikama (1973) recorded the occurrence of this species from the (?) Late Miocene Zushi Formation of Miura Peninsula. Though I have inquired at the store room of the Yokohama National University where the collection related to this paper is supposed to be preserved, I could not find the relevant specimens. Aside from this occurrence, hitherto known stratigraphic occurrences of C. yanagawaensis seem to be restricted to the Middle Miocene in north and central Honshu. Several specimens from the Akeyo Formation in the Mizunami area and the Sunakozaka Formation in the Kanazawa area are undoubtedly referable to this species.
Aequipecten matsunagiensis Masuda, 1966, from the (?) Middle Miocene Higashi-innai Formation in Ishikawa Prefecture (central Honshu), is probably not a representative of Cryptopecten, because the irregularly bifurcated radial ribs in the holotype (Masuda, 1966, pl. 35, fig. 4) are not to be found in this genus. I think that this species is rather closely related to Chlamys otukae Masuda and Sawada, 1960. However, one of the paratypes of A. matsunagiensis (Masuda, 1966, pl. 35, fig. 6) shows regular and simple radial ribs which remind me of those of C. yanagawaensis.
The present species resembles C. bullatus in the large number of radial ribs on the disk, comparatively weak shell convexity and low outline. In some other characteristics, especially in the considerably thick test, flat-topped central ridge of each rib and oppositely arranged imbricated scales, it is rather similar to Phenotype Q of C. vesiculosus. In these respects, C. yanagawaensis is morphologically intermediate between the two living species but clearly distinguishable from them.
Distribution. —This species is known from the Yanagawa Formation at Yanagawa, Fukushima Prefecture (type locality), the Moniwa Formation at Moniwa and Jûnishin (Masuda, 1958), Taira (Masuda and Takegawa, 1965) and Kanagase of Ôgawara (IGPS no. 90785) near Sendai, Miyagi Prefecture, the Akeyo Formation (Shukunohora Sand stone) at Mizunami and Nataki (Itoigawa, Shibata and Nishimoto, 1974), Gifu Prefecture, and the Sunakozaka Formation at Higashi-ichise near Kanazawa (Ogasawara, 1976), Ishikawa Prefecture. Early Middle Miocene (15-16 Ma).
|
* The suprafamilial classification by Waller (1978) is here adopted.[return to the text] * There are several quite different opinions about the contents of the genus (or subgenus) Argopecten, including the nature of its type-species, Pecten solidulus Reeve, 1853. Though I am not in a position to discuss this nomenclatorial problem, I here follow Waller's (1969, pp. 32-34) interpretation, in which the type specimen of P. solidulus is referable to Pecten circularis Sowerby, 1835, from the eastern Pacific (not to such Mediterranean species as Pecten commutatus Monterosato, 1875); and, therefore, Plagioctemum Dall, 1898, should be treated as a junior synonym of Argopecten.[return to the text] |