Phylogenetic Significance of the Lateral Line System in the Family Cyprinidae
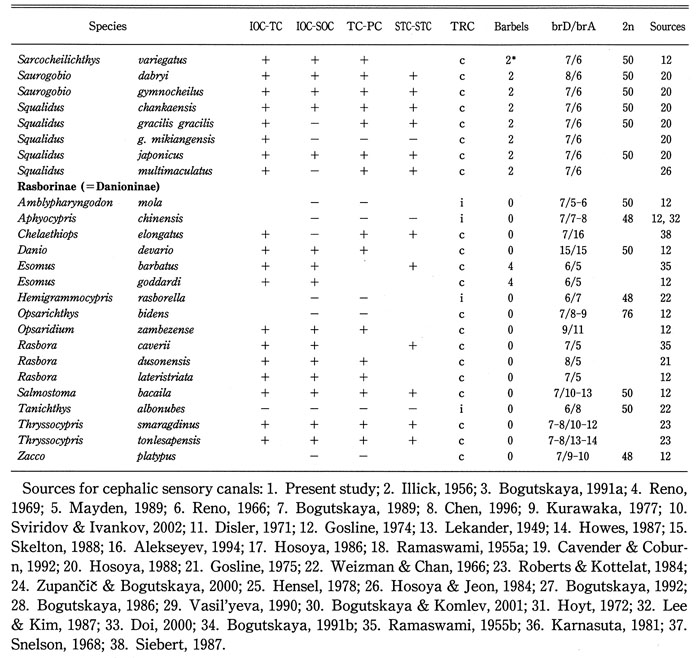
Distribution of cephalic sensory canals, trunk canal (TRC), barbels, branched fin-rays of dorsal and anal fins (brD/brA), and diploid chromosome number in 132 (63% of total) genera of the family Cyprinidae were analyzed as shown in Table 2, and summarized in Table 3. Barbels were selected as representative of the sensory organ, branched fin rays of the dorsal and anal fins as an important factor for swimming, and the diploid chromosome number as genetic taxonomic tool. Data on diploid chromosome numbers were extracted from Amemiya & Gold (1987), Arai (1982), Gold et al. (1990), Klinkhardt et al. (1995), Lee et al. (1986), Tsigenopoulos et al. (2002), Ueda et al. (1997, 2001), Ueno et al. (1992), and Yu et al. (1989). From this analysis, the following points could be made:
- Table 2.
-
Distribution of the cephalic sensory canal system, the trunk canal, barbels, branched fin-rays of dorsal and anal fins (brD/brA), and diploid chromosome number in the family Cyprinidae.
Generic names in each sub family arranged alphabetically. BrD/brA shows number; IOC-TC, continuity between IOC and TC; IOC-SOC, continuity between IOC and SOC; TC-PC, continuity between TC and PC; STC-STC, continuity between right and left STCs. Continuity (+), discontinuity (-), complete (c), and incomplete (i), incomplete infraorbital canal (+*), absence of supratemporal canal (-*), absence of trunk canal (i*). 2* and 0* in the barbel column mean a minute barbel at the tip of the maxilla and a fleshy barbel in the groove between the maxilla and eye, respectively. Blanks mean unknown.
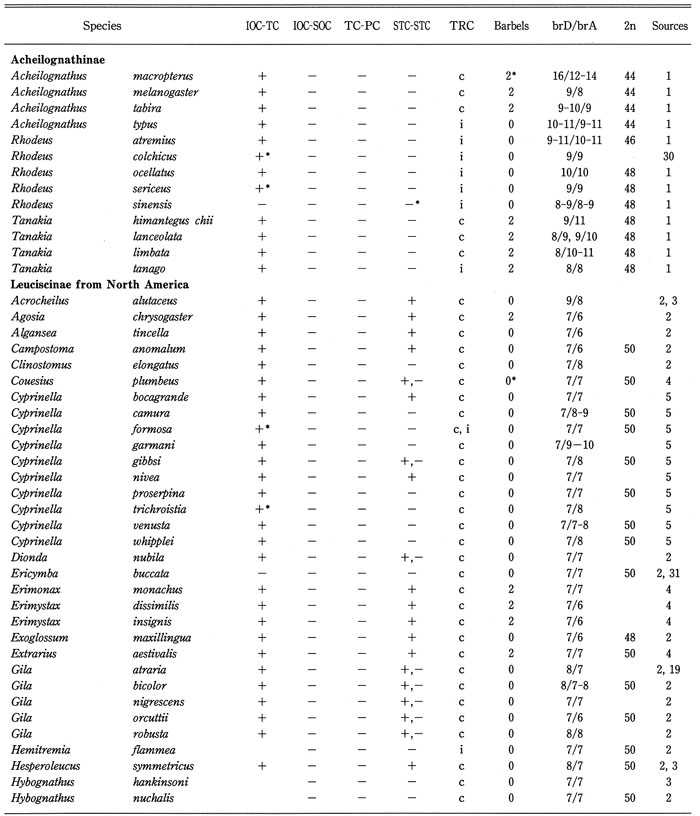
Table 2. (Continued)
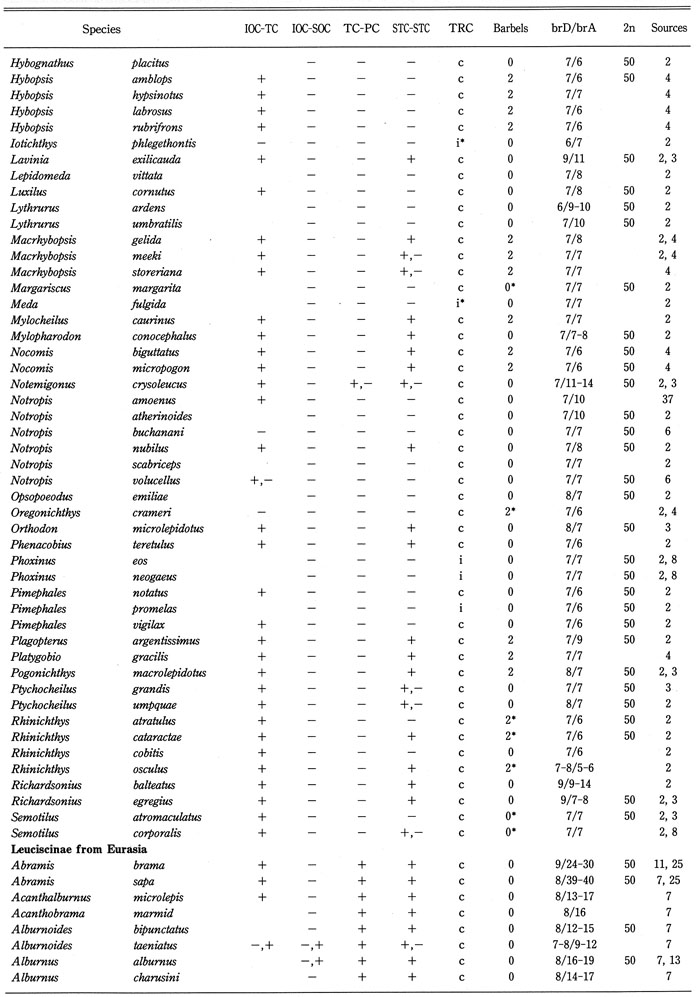
Table 2. (Continued)
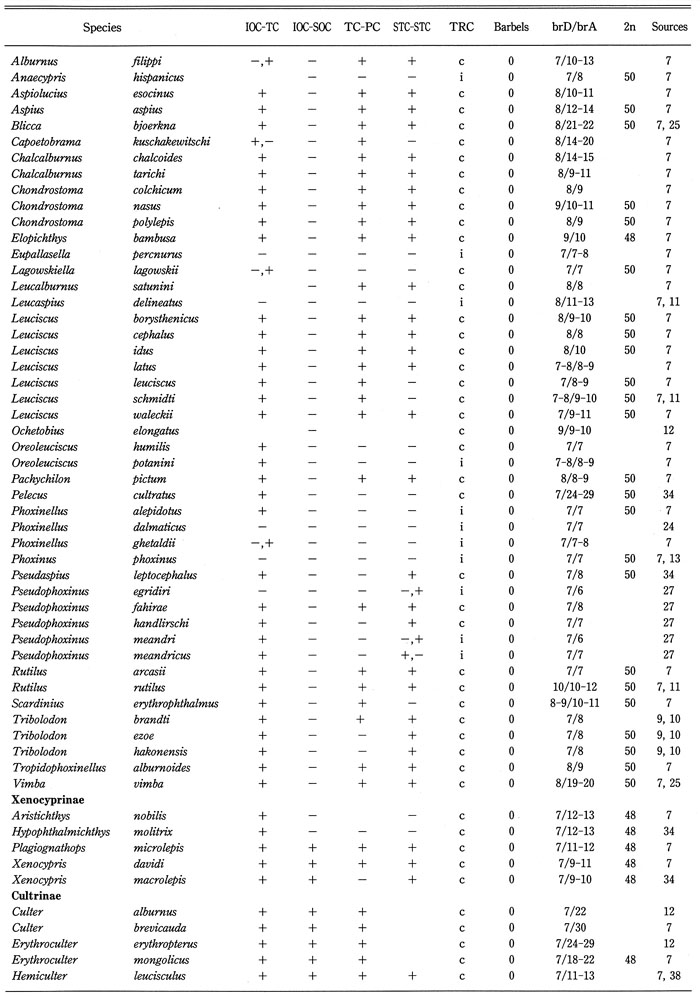
Table 2. (Continued)
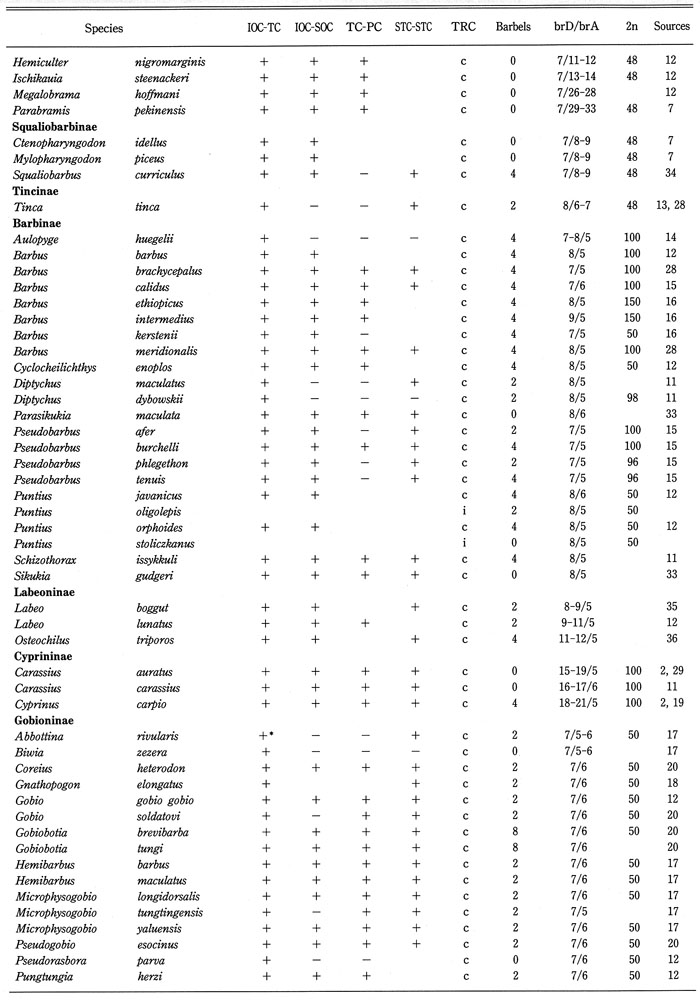
Table 2. (Continued)
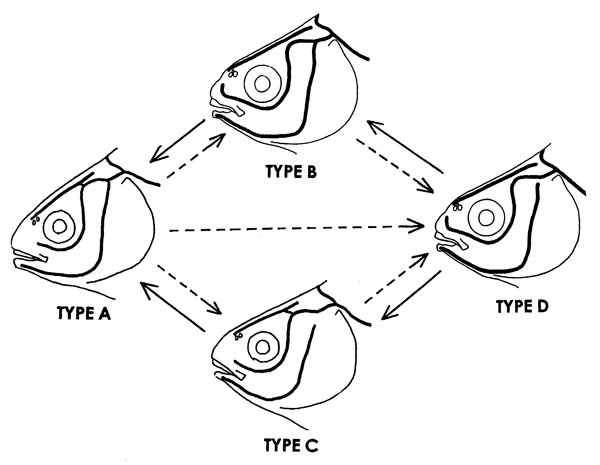
(1) Based on continuity or discontinuity between IOC and SOC (IOC-SOC), and between TC and PC (TC-PC), the cephalic sensory canal system in the Cyprinidae could be divided into four types, Types A, B, C, and D as shown in Fig. 15. Type A was defined by continuity of both IOC-SOC and TC-PC, Type B by discontinuity of IOC-SOC and continuity of TC-PC, Type C by continuity of IOC-SOC and discontinuity of TC-PC, and Type D by discontinuity of both IOC-SOC and TC-PC. Types I, II, and III canal systems in the Acheilognathinae (Fig. 1) belong to Type D, and are characterized by discontinuity of STC-STC.
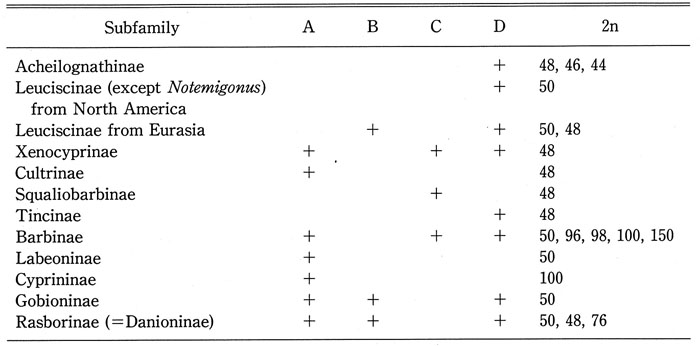
(2) As for distribution of Types A to D in cyprinid subfamilies, Acheilognathinae, North American Leuciscinae (except Notemigonus), and Tincinae were characterized by only Type D; Eurasian Leuciscinae by Types B and D; Squaliobarbinae by Type C; Cultrinae and Cyprininae by Type A; Gobioninae and Rasborinae (=Danioninae) by Types A, B, and D; Xenocyprinae and Barbinae by Types A, C, and D. There were no sub families having both Types B and C.
(3) Type D corresponds to a structure at juvenile stage in cyprinids having Type B, C or A. Type B or Type C corresponds to a structure at juvenile stage in cyprinids having Type A. There were no subfamilies having both Types B and C. Therefore, as shown in Fig. 15, at least two ontogenetic pathways (developmental sequences) of cephalic sensory canals were hypothesized, i.e., Type D → Type → Type A, and Type D → Type C → Type A (Disler, 1971; Hosoya, 2001; Lekander, 1949; Smith, 2001): the Leuciscinae from Eurasia, the Gobioninae, and the Rasborinae, which shared Type B, may belong to a different clade from the three subfamilies, Barbinae, Squaliobarbinae, and Xenocyprinae, which shared Type C. From the viewpoint of character phylogeny of the cephalic sensory canal system, Types B, C, and D might have derived from Type A. Type D might have derived from Type B or Type C as well as Type A. Therefore, it could not be concluded that the three subfamilies, the Acheilognathinae, Leuciscinae (except Notemigonus) from North America, and Tincinae, in which only Type D could be found, were closely related to each other (Alberch et al., 1979; Fink, 1982; Webb, 1989a, 1989b) (Fig. 15).
(4) From the fact that the sensory canal system in all sexually mature bitterlings corresponds to a structure at juvenile stage in any other cyprinid subfamily fish, in which the right and left supratemporal canals are continuous, it was hypothesized that the Acheilognathinae might have evolved by peadomorphosis from an ancestor of this subfamily (Disler, 1971; Hosoya, 2001; Lekander, 1949).
(5) For species with an incomplete trunk canal, the IOC-SOC, TC-PC and STC-STC were all discontinuous. From this fact, it was confirmed that an incomplete trunk canal state in adults could be a good marker on peadomorphosis.
(6) Continuity of IOC-TC was the most conservative among those between cephalic sensory canals, i.e., all species having a continuous IOC-SOC or TC-PC were characterized by continuity of IOC-TC. From this fact it was predicted that any species, in which continuity of IOC-SOC or TC-PC was examined but continuity or discontinuity of IOC-TC was unknown, was characterized by continuity of IOC-TC.
(7) Cavender & Coburn (1992) and Chen et al. (1984) reported a sister group relationship between the Acheilognathinae and the Gobioninae. However, such a sister group relationship is problematic by the following reasons. Cavender & Coburn (1992: 295) listed 3 characters (Characters 17, 40, and 43) as synapomorphies of the Acheilognathinae and the Gobioninae. However, Characters 17 and 40 (Cavender & Coburn, 1992: 323-324) were incorrectly analyzed, because both plesiomorphic and apomorphic states were found in the Gobioninae, i.e., the plesiomorphic state was found in Hemibarbus, Gobio, and Saurogobio (Hosoya, 1988). As regards the cephalic sensory canal system, the Gobioninae exhibited Types A, B, and D, but the Acheilognathinae, only Type D. Type A in the Gobioninae suggests that the Acheilognathinae was not more closely related to the Gobioninae than to the Leuciscinae (Fig. 15). The subfamily Gobioninae as defined by Banarescu & Nalbant (1973), Chen et al. (1984), and Yue (1998) was diagnosed by 7/6 in branched dorsal fin rays/branched anal fin rays, no spinelike simple ray in the dorsal fin, two barbels, and a complete trunk canal. As shown in Table 2, these characters were shared by North American leuciscine genera such as Erimystax, Nocomis, and Oregonichthys, which were classified in the tribe Phoxinin by Cavender & Coburn (1992). Hosoya (1986) recognized the Gobioninae sensu Banarescu & Nalbant (1973) as polyphyletic, and excluded the eight genera, Pseudorasbora, Pungtungia, Pseudopungtungia, Coreoleuciscus, Sarcocheilichthys, Ladislavia, Gnathopogon, and Coreius, from the Gobioninae. A sister group relationship between the Gobioninae sensu Hosoya (1986) and the Cyprininae has been suggested (Arai, 1982; Hosoya, 2001). Type D of the cephalic sensory canal system and two barbels were shared by the Acheilognathinae and the North American barbelled Leuciscinae, which differed from the Eurasian Leuciscinae by absence of Type B and presence of barbels. These points mean that it will be necessary to retest the monophyly of the Gobioninae and the Leuciscinae before a sister group relationship between the Acheilognathinae and the Gobioninae can be analyzed.
- Table 3.
-
Summary of Table 2, showing four types (A, B, C, and D) of the cephalic sensory canal system and diploid chromosome number in the family Cyprinidae.
For explanation of four types, see text and Fig. 15.
- Fig.15.
- Four types (Types A, B, C, and D) of the cephalic sensory canal system in the Cyprinidae. Arrows in solid line show morphogenesis. Arrows in dashed line show evolution by peadomorphosis. (Figures of Types B and D from Hensel, 1978)