Cephalic Sensory Canals and Canal Pore Counts
Generally, SOC, IOC, TC, MC, STC, and PC connect in basal cyprinids (Gosline, 1974), and therefore, this type of cephalic sensory canal system (Type A in Fig. 15) has been considered plesiomorphic in the family Cyprinidae (Gosline, 1974; Webb, 1989b). On the other hand, Bogutskaya (1989, 1991b) hypothesized that the absence of a connection between IOC and SOC (IOC-SOC) (Types B and D in Fig. 15) was a plesiomorphic state in the Cyprinidae. However, her hypothesis was not adopted here because, based on morphology and karyotypes, a discontinuity of IOC-SOC was restricted to Eurasian Leuciscinae and most specialized subfamilies such as Acheilognathinae, Tincinae, and North American Leuciscinae, while a continuity of IOC-SOC (Type A in Fig. 15) was found in most basal subfamiles such as Barbinae and Rasborinae (Tables 2 and 3).
In bitterlings, the cephalic sensory canal system was characterized by discontinuity between IOC and SOC, PC and TC, and between right and left STCs. Both continuity and discontinuity between right and left STCs has been reported in the Leuciscinae, Xenocyprinae, Barbinae, Gobioninae, and Rasborinae (Table 2). However, the Acheilognathinae was only sub family, all species of which lacked the connection between the right and left STCs. Therefore, this character was considered as an autapomorphy of the sub family Acheilognathinae (Fig. 1). A discontinuity of IOC-SOC was found in the Acheilognathinae, Leuciscinae, Xenocyprinae, Tincinae, Barbinae, Gobioninae, and Rasborinae (Table 2). Although Gosline (1974) reported connection between IOC and SOC in Acheilognathus intermedia (=T. lanceolata) and Paraheilognathus rhombea (=A. rhombeus), and Meng (1985: 25, fig. 5) figured a connection between SOC and TC as well as connection between right and left STCs in Acheilognathus taenianalis, we have found neither a connection between IOC and SOC nor a connection between right and left STCs in any species of bitterlings. Cavender & Coburn (1992) reported the absence of the canal on the parietal bone in the Acheilognathinae, but in our study, the canal was found in Tanakia limbata, T. tanago, and all species/subspecies of Acheilognathus except A. melanogaster. In Tanakia lanceolata and A. melanogaster, SOC extended posteriorly to the pterotic bone. Discontinuity of TC-PC has been reported in the Leuciscinae, Xenocyprinae, Squaliobarbinae, Tincinae, Barbinae, Gobioninae, and Rasborinae (Bogutskaya, 1986, 1989; Cavender & Coburn, 1992; Hosoya, 1986; Illick, 1956; Mayden, 1989; Reno, 1966, 1969) (Table 2).
The phylogenetic polarity of cephalic sensory canal character states has been proposed in teleostean fishes by a number of earlier researchers as follows: complete IOC is plesiomorphic (Gosline, 1974; Webb, 1989b); presence of TC and STC, plesiomorphic (Gosline, 1974; Webb, 1989b). To determine the polarity of IOC, TC, and STC in bitterlings, their states were mapped on a molecular tree based on 12S ribosomal DNA sequences (Fig. 13). This confirmed the polarity as described by Gosline (1974) and Webb (1989b). All types of the cephalic sensory canal system (Types I, II, and III) in the sub family Acheilognathinae were found in Rhodeus species having 48 diploid chromosomes (Tables 1 and 2, Fig. 1). Types II and III were found in R. sericeus and R. sinensis, respectively, and Type I in other Rhodeus species/subspecies. Bogutskaya & Komlev (2001) reported Type II in Rhodeus sericeus amarus and R. colchicus from West Transcaucasia. Type I, which is characterized by complete IOC (plesiomorphic) combined with presence of TC and STC (plesiomorphic), is plesiomorphic, while Types II and III are apomorphic in bitterlings. As character combinations of Types II and III are contradictory (in Type II, TC and STC are plesiomorphic but IOC is apomorphic vs. in Type III, TC and STC are apomorphic but IOC is plesiomorphic), it was hypothesized that R. sericeus (Type II) and R. sinensis (Type III) might have evolved separately from an ancestor of Rhodeus (Type I) (Fig. 13). An incomplete IOC has been reported in the Leuciscinae as well as the Acheilognathinae (Bogutskaya, 1992; Mayden, 1989) (Table 2).
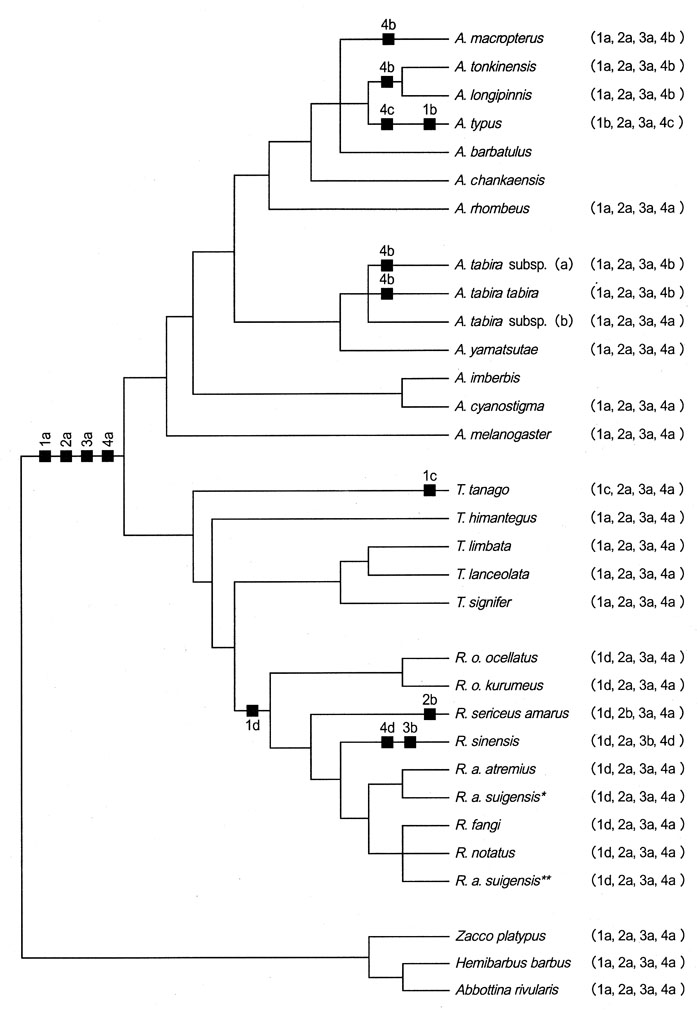
- Fig.13.
- Mapping of the lateral line system onto the most parsimonious strict consensus tree based on 12S ribosomal DNA sequences. This tree is modified from Okazaki et al. (2001) by combining plural branches of different localities of a species into one branch. '★'specimen from Japan; '★★'specimen from China. Character 1 refers to the trunk canal states: 1a, complete TRC; 1b, incomplete TRC in A. typus; 1c, 5-20 pored scales; 1d, 0-8 pored scales. Character 2 refers to IOC: 2a, complete; 2b, incomplete. Character 3 refers to TC and STC: 3a, presence; 3b, absence. Character 4 refers to the number of infraorbital bones: 4a, 5; 4b, 6; 4c, 7; 4d, 4.
Lekander (1949) studied development of sensory canals in Leuciscus rutilus (= Rutilus rutilus) and reported that the separated canals at an early juvenile stage became connected to each other as development proceeds. The cephalic sensory canals were formed in an order of PC, SOC, IOC, STC, and TC. Such order of morphogenesis in the cephalic sensory canal system has been also reported in barbine, gobionine, and leuciscine cyprinids (Disler, 1971; Hosoya, 2001). From these facts, it was considered that the cephalic sensory canals of R. sinensis corresponded to those at a juvenile stage of Type I Rhodeus bitterling, suggesting that R. sinensis might have evolved from a Type I Rhodeus bitterling by peadomorphosis (Alberch et al., 1979; Fink, 1982; Gould, 1977; Webb, 1989b).
It was difficult to find differences in sensory canal pore counts among Tanakia, Acheilognathus, and Rhodeus in the number of canal pores of SOC, IOC+TC, MC+PC and/or STC. This might become discernible if a great number of specimens were to be examined.