V. Discussion
V-1. Relationships of Major Archaeogastropod SubgroupsArchaeogastropoda Thiele, 1925: From the results of this study, the following comments are proposed on the status of Archaeogastropoda and Architaenioglossa: (i) Archaeogastropoda is defined as a "patellogastropod clade+rhipidoglossate clade," namely as a "pre-taenioglossate grade." (ii) "Archaeogastropoda" is undoubtedly a paraphyletic taxon, and therefore should not be used as a formal taxon name in phylogenetic classification. However, as a long-established group not easily discarded, "Archaeogastropoda" is retained as a collective name for gastropods with primitive radular morphology and buccal mass structure, (iii) Architaenioglossa is independent from the "Archaeogastropoda" and belongs to the Caenogastropoda clade. The hypoathroid condition is a plesiomorphic state of Gastropoda, and accordingly, it should not be overly stressed. Caenogastropoda is defined by synapomorphies such as a taenioglossate radula, compact buccal musculature, distinct esophageal structure, and a monopectinate ctenidium without bursicles. (iv) Neotaenioglossa is separated from Architaenioglossa by synapomorphies such as loss of the radular diverticulum, retractor muscles of the odontophore, and an epiathroid nervous system. Patellogastropoda Lindberg, 1986: The monophyletic separation of Patellogastropoda (node 4) from other gastropods was strongly supported in this study by unambiguous synapomorphies such as retractile circumpallial tentacles (character #2), a transverse labial muscles (#26), dorsal protractors of the odontophore (#27), anterior band of the jaw (#37), anterolateral cartilages (#55), a two-layered ventral approximator muscle (#57), a septate mid-esophagus (#63), a muscular bulbous aorta (#69), and labial ganglia (#86). Other suggested synapomorphies include osphradia on the floor of the pallial cavity (#22), teeth mineralization (#45) and a simplified stomach (#65) by ACCTRAN optimization, and eyes within the cephalic tentacle (#6), double-layered structure of the jaw attaching to buccal mass (#35, 36), paired kidneys on the right side of the pericardium (#72, 73) by DELTRAN optimization. Division of the shell muscle (#16), ventral position of the gonad (#75), and connection of the gonoduct with the kidney (#77) are unambiguous synapomorphies, but homoplasies with other clades. The loss of the ctenidium (#17) occurred prior to the split of Patellogastropoda. In relationship to other gastropods, Patellogastropoda are regarded as the earliest gastropod offshoot in accordance with recent theories (Ponder and Lindberg, 1996; 1997). Their suggested position has been based mainly on the stereoglossate radula and symmetrical limpet-shaped shell without helical coiling of teleoconch, both of which are shared with Tryblidiida (Golikov and Starobogatov, 1975; McLean, 1979; Salvini-Plawen, 1981; Wingstrand, 1985; Salvini-Plawen and Haszprunar, 1987; Haszprunar, 1988 a, b). In addition, the basal position of Patellogastropoda has also been suggested by the shallow mantle cavity, ctenidium without skeletal rods, paired excretory system, simple eye type, position of statocysts, lowest number of chromosomes (n= 9-10) (Haszprunar, 1988 b), primitive-type sperm morphology (Koike, 1985; Healy, 1988; 1996), and an osphradium lacking true sensory cells (Haszprunar, 1985 a). Furthermore, the double-layered jaw plate shared by Patellogastropoda and Cephalopoda possibly supports primitiveness of Patellogastropoda (see section V-2). Thus, anatomical characters strongly support the basal position of Patellogastropoda. Orthogastropoda Ponder and Lindberg, 1996: All remaining non-patellogastropod groups (node 9) are here regarded as a clade. Monophyly of this large group is supported by unambiguous synapomorphies such as eyes with a vitreous body (#8), unpaired osphradium (#21), paired jaws (#34), the position of the jaws free from the buccal mass (#36), and a flexoglossate radula (#44), the elongation of marginal teeth (# 53), and a single kidney on the right side of pericardium (#72, 73). In addition, eyes outside bases of cephalic tentacles (#6), eyestalks (#7), skeletal rods, (#19), lateral ciliated zones of osphradium (#24), a single left hypobranchial gland (#25), single-layered jaws on oral tube (#35, 36), and 4 pairs of lateral teeth (#49) by ACCTRAN and an unpaired ctenidium (#17), the absence of teeth mineralization (#45), a style sac region in the stomach (#65) by DELTRAN are possible synapomorphies that discriminate this clade from patellogastropods. The Orthogastropoda is further divided into rhipidoglossate and non-rhipidoglossate clades. These rela- tionships are greatly different from those of recent studies (Haszprunar, 1988 a, b; Salvini-Plawen and Steiner, 1996; Ponder and Lindberg, 1996, 1997). The separation of caenogastropod genera (node 24) from rhipidoglossate archaeogastropods is supported by unambiguous synapomorphies such as monopectinate ctenidial filaments (#18), the absence of anterior levator muscles (#28), a single pair of lateral teeth (#49), and posteriorly separated esophageal pouches (#62). Eyes outside of cephalic tentacles (#6), eyestalks (#7), skeletal rods (#19), lateral ciliated zones of osphradium (#24), a single left hypobranchial gland (#25) are all homoplastic with some rhipidoglossate taxa by DELTRAN optimization. Rhipidoglossa Mörch, 1865: In contrast to recent theories, rhipidoglossate groups (node 10) form a distinct clade. Its monophyly is justified by unambiguous changes such as the position of the osphradium associated with the ctenidial membrane and axis (#22), numerous pairs of marginal teeth (#52), the loss of salivary gland (#61), the presence of a gastric caecum (#64), and statocysts on the anterolateral sides of the pedal ganglia (#93). Other possible synapomorphies for this clade include the postdorsal buccal tensor (# 32) and outer approximator (#58) muscles in ACCTRAN optimization, and four pairs of lateral teeth (#49) in DELTRAN optimization. Neritopsina Cox and Knight, I960: The result of the present cladistic analysis strongly supported the robust monophyly of neritopsine groups (node 21) that are diversified from marine to terrestrial habitats. Although some external characters are secondarily modified according to various life habits, they retain astonishingly common organization in the internal organs, especially in buccal musculature, odontophoral cartilages, the anterior alimentary tract, and nervous system. Synapomorphies with unambiguous changes include the dorsal levator (#29) and posterior depressor (#31) muscles, the median cartilages (#56), the tensor muscle of the anterior cartilages (#59), posterior esophageal glands (#63), a diaulic female gonoduct (# 79). Enlargement of the fourth lateral teeth (#50), the presence of posterior cartilages (#54), origin of the visceral loop from the right side only (#90) are all homoplastic with some other groups. Also, the absence of ctenidial rods (#19), and the hypobranchial gland on the right side (#25) in ACCTRAN, and the postdorsal buccal tensor (#32) and outer approximator (#57) muscles in DELTRAN may be independently acquired in this clade. Concerning the systematic position of Neritopsina within "Archaeogastropoda," many authors (e.g. Yonge, 1947; Cox, 1960; Fretter, 1965, 1984 a; Graham, 1985) have historically favored an intermediate position between primitive rhipidoglossate groups and Caenogastropoda based on the closed pallial gonoduct and single left kidney (="loss of right one"). Meanwhile, Haszprunar (1988 a, b) advocated its more basal position between Vetigastropoda and Cocculiniformia, emphasizing the primitive state of the ctenidium without skeletal rods. The results of the present cladistic analysis did not support such a basal position, but indicated more intimate relationships with other rhipidoglossans than with any other taxa. "Non-neritopsine Rhipidoglossa": The unity of this group (node 11) is suggested by unambiguous syn- apomorphies such as epipodial tentacles (#12), a bifurcated posterior end of the radular sac (#38), and absence of a labial commissure (#87). Absence of a ciliated zone in the osphradium (#23) (in ACCTRAN) and the presence of skeletal rods (#19) (in DELTRAN) may have uniquely evolved in this clade. This clade is equivalent to Archaeogastropoda (s.s.) as restricted by Ponder and Lindberg (1997). As in their analysis, this clade was weakly supported in this study. "Vent Taxa" = Peltospiroidea McLean, 1988+Neomphaloidea McLean, 1981: Among specialized taxa specific to a deep-sea environment, Neomphaloidea and Peltospiroidea are not closely related with other traditionally known archaeogastropods. Since their discovery in the 1980 s, they have been variously allocated to positions around Vetigastropoda as paraphyletic taxa (Salvini-Plawen and Haszprunar, 1987; Haszprunar, 1988 a, b; Salvini-Plawen and Steiner, 1996). Their relationships were also poorly resolved in the cladistic analysis of Ponder and Lindberg (1997). At first sight these vent taxa seem related to "higher" gastropods because they share certain "advanced" gastropod characters, such as a single left auricle, a ventricle independent of the rectum, and a gonoduct independent from the right kidney (Fretter, 1989). On the other hand, characters such as the rhipidoglossate radula, buccal musculature, and the anterior loop of the intestine suggest their affinities to other rhipidoglossate groups of "Archaeogastropoda." Although their systematic positions are still uncertain, they are probably associated with Vetigastropoda according to an osphradium associated with the efferent ctenidial axis, enlarged esophageal pouches with papillae glands, and the postmedian retractor muscle of the radula (in Neomphalus). At present, these two specialized superfamilies can be preliminarily grouped as a vernacular name, "vent taxa." The resolution of these groups is an important subject for further studies in gastropod phylogeny. "Cocculiniformia" Haszprunar, 1987: This taxon was proposed for deep-sea limpets with a secondary respiratory organ (a pseudoplicate gill or several pallial/subpallial leaflets), a modified rhipidoglossate radula, and a specialized feeding mode in the deep-sea environment (Haszprunar, 1987 a, 1988 c; see Appendix). The characteristic reproductive system configuration (hermaphroditic gonad and copulatory organ on the right side) is also assumed to support the monophyly of this taxon (Salvini-Plawen and Haszprunar, 1987; Haszprunar, 1988 c). Members of this group were allocated to a position between the Patellogastropoda and Neritopsina (first offshoot of Orthogastropoda), emphasizing the primitiveness of limpet-like forms with a shallow mantle cavity and divided shell muscle (also shared with Pateliogastropoda) (Saivini- Plawen and Haszprunar, 1987; Haszprunar, 1988 a, b; Salvini-Plawen and Steiner, 1996). In contrast to these considerations, the monophyly of Cocculiniformia was clearly rejected by the cladistic analysis of Ponder and Lindberg (1996). Cocculinidae were united with Neritopsina, while Lepetelloidea were allocated to a first offshoot of Vetigastropoda. They assumed that the modifications (mostly reduction) were caused by paedomorphosis. (1) Monophyly of Cocculiniformia: As discussed by Ponder and Lindberg (1997), characters listed as synapomorphies for Cocculiniformia by Haszprunar (Haszprunar, 1987 a, 1988 c) are, in fact, not truly specific to this group, nor universally shared within the group. For example, the absence of (post-torsional) right pallial organs and a monotocardian heart are not diagnostic only for this group. Possible synapomorphies are the hermaphroditic reproductive system and a copulatory organ derived from the right side. Some similarities between Cocculinoidea and Lepetelloidea also found in other taxa of archaeogastropods as synplesiomorphies, and accordingly do not support their monophyly. (2) Cocculinoidea-Neritoidea: According to analysis of Ponder and Lindberg (1996, 1997), Cocculinidae and Neritidae are united by synapomorphies such as 1) a divided shell muscle, 2) absence of salivary glands, 3) jaw structure, 4) sublingual glands, 5) enlarged fourth lateral teeth, 6) a shift in the position of the anus from the posterior half to the anterior half of the pallial cavity, and 7) absence of the right kidney (in contrast to that in Lepetelloidea). In addition, internal copulation, a single ctenidium (if the pseudoplicate gill is homologous with a ctenidium), and absence of skeletal rods in the gills do not seem to contradict this relationships. Based on my own observations, however, these characters do not plausibly suggest the close relationships of these two taxa: (i) Jaw structure is neither unique to these two nor similar between them. (ii) Sublingual glands projecting from the sublingual pouch are very prominent in neritoid genera, but are not found in Cocculina nipponica in comparable form. These results contradict Ponder and Lindberg's (1996; 1997) character coding. Meanwhile, it is possible that the enlarged outer lateral (fourth) teeth and division of the shell muscle by blood vessels may have evolved in the common ancestor of these taxa. Therefore, only the latter two characters can support the monophyly of Cocculina and Nerita. In buccal characters there is no evidence for close relationship. (3) Lepetelloidea-Fissurelloidea: Positive evidence was presented for a relationship between these two groups by Ponder and Lindberg (1996; 1997). The fourth lateral teeth are markedly enlarged in Cocculinoidea, whereas in the Pseudococculinidae it is the fifth lateral teeth as in Fissurellidae. In addition, some states found in Lepetelloidea (two kidneys, and rectum penetrating ventricle) are shared with Vetigastropoda rather than with Cocculinoidea (see Table 4). Thus, it may not be unlikely that Lepetelloidea are greatly modified vetigatropods, although the relation is not very robust (Ponder and Lindberg, 1997). (4) Cocculiniformia-Vetigastropoda: In contrast to previous works, Cocculina is more closely related to Vetigastropoda than to Neomphalus (vent-taxa) and Neritopsina in this study. These relationships (node 12) are directly supported by the presence of a dorsal buccal tensor (#33) and a median tensor of the radular sac (#42). Postdorsat buccal tensor (#32) and outer approximator (#58) muscles are also possible synapomorphies for this clade in DELTRAN optimization. If this position is true, all cocculiniform groups, including Cocculinoidea and Lepetelloidea, may form a sister group with Vetigastropoda, independent of Neritopsina. However, as the robustness of this clade is not very high, this is only one of several possibilities. Vetigastropoda Salvini-Plawen, 1980: The name Vetigastropoda has previously been used for Zeugo- branchia+Trochoidea+Cocculinoidea (Salvini-Plawen, 1980) or Lepetodriloidea+Zeugobranchia+Trochoidea (Haszprunar, 1988 a, b; Salvini-Plawen and Steiner, 1996). The definition is here extended to include Seguenzioidea+Lepetodriloidea+Zeugobranchia+Trochoidea in agreement with Ponder and Lindberg (1997). These taxa (node 13) are united by many synapomorphies, such as epipodial sense organs (#13), fimbriate anterior edge of the jaw (#37), expanded esophageal pouches (#62), papillate esophageal glands (#63), paired kidneys on either side of pericardium (#72, 73). In addition, micropapillae on the tentacles (#14), the posterior depressor muscles (#31), five pairs of lateral teeth (#49), paired auricles (#67), a transverse pallial vein (#71) are also possible synapomorphies in ACCTRAN optimization. (1) Zeugobranchia + Trochoidea: This clade (node 15) is united by unambiguous synapomorphies including the osphradium on the free tip of the ctenidium (#22), paired hypobranchial glands (#25), ramified salivary glands with slit-like openings into the buccal cavity (#61), and presence of a papillary sac (#74). Bursicles (#20) and paired postmedian retractor muscles of the radular sac (#42) occur independently in this clade under DELTRAN optimization. (2) Zeugobranchia: Zeugobranchs (node 16) uniquely share two unambiguous synapomorphies, a median mantle slit (#1) and paired ctenidia (#17). Paired osphradia (#21), asymmetry of the radular teeth (#47) and enlargement of the fifth outer lateral teeth (#50), and basibranchial sinus (#70) are also specialized within this clade in ACCTRAN optimization. Within Zeugobranchia, four superfamilies (Pleurotomarioidea, Haliotoidea, Fissurelloidea, and Scissurellidae) are recognized as extant forms. They share the following character states: (i) Pleurotomariidae and Haliotidae are linked by open eyes (#8), unenlarged outer lateral teeth (#51), and a spiral gastric caecum (# 63) originating from the large U-shaped stomach. They differ in external anatomy (epipodial tentacles, cephalic lappets, eye stalks) and radular morphology (hystricoglossate vs. typical rhipidoglossate). (ii) Fissurellidae and Scissurellidae are related by closed eyes (#8) and a short gastric caecum (#63), but differ in symmetry of the radular teeth (#47), morphology of the epipodial tentacles, presence or absence of micropapillae (#14), and the pedal nervous system (#91, 92). (iii) Pleurotomariidae, Haliotidae, and Fissurellidae share asymmetrical radular teeth (#47) only, although their radular morphologies are distinctly different. (iv) Haliotidae and Scissurellidae have pallial tentacle(s) (#3). (v) Other combinations of the four families reveal no shared character states. Recently Harasewych et al. (1997) discussed the position of Pleurotomariidae based on cladistic analyses using 18 S rDNA. Their results indicate that Pleurotomariidae is split first within Vetigastropoda and non- pleurotomarid zeugobranchs plus Trochoidea form another clade. This relationship is not consistent with the results of this study in which zeugobranchs form a clade. (3) Trochoidea: The monophyly of Trochoidea (node 19) is supported by unambiguous synapomorphies of neck lobes (#10), and protolateromarginal radular plates (#51). The relationships within trochoidean families, Turbinidae, Trochidae, and Skeneidae, are not clearly established, but Turbinidae was regarded as most primitive by Hickman and McLean (1990) and Hickman (1996). The distinction between Turbinidae and Trochidae is given primarily by basal morphology of central tooth of the radula, and therefore, Trochidae is diagnosed as a "non-turbinid" group (Hickman and McLean, 1990). (4) Lepetodriloidea: Lepetodriloidea is more closely related to "Zeugobranchia+Trochoidea" than to Seguenzioidea. Unambiguous synapomorphies for "Lepetodrilus+Zeugobranchia+ Trochoidea" (node 14) are in having the rectum penetrating the ventricle (homoplasy with Neritoidea) (#67), paired auricles (#68), and a connection of the gonoduct with the kidney (homoplasy with Patellogastropoda) (#77). This relationship was also supported by the posterior depressor muscle (#31) and five pairs of lateral teeth (#49) in DELTRAN optimization. Paired kidneys with renopericardial ducts, papillate esophageal glands, and epipodial sense organs are also general vetigastropod characters, Thus, inclusion of this taxon in Vetigastropoda is confirmed, as discussed by Haszprunar (1988 a, b), Fretter (1989), and Ponder and Lindberg (1997). (5) Seguenzioidea: Historically, this family has been variably treated by different authors. (i) It was assigned to "Mesogastropoda" based on the taenioglossate-like radula with a single pair of lateral teeth (e.g. Golikov and Starobogatov, 1975). (ii) It was separated from other gastropod groups as a distinct archaeogastropod taxon 'Seguenziina' by Salvini-Plawen and Haszprunar (1987) and Haszprunar (1988 a, b), stressing the peculiarity of the internal structure of the esophagus. (iii) It was regarded as "archaeogastropods with trochoidean affinity" based on the trochispiral shell form, a nacreous shell layer, a trochoid-like protoconch, epipodial tentacles, and an anterior loop of the intestine (Bandel, 1979; Quinn, 1983). Also in the phylogenetic scheme of Ponder and Lindberg (1996, 1997), the family was considered closely related to Trochoidea. The present phylogenetic analysis suggests that the family can be placed within Vetigastropoda because of possession of papillate epipodial tentacles (#14), bursicles (#20), and slightly papillate esophageal gland in enlarged esophageal pouches (#62, 63). In contrast, the monopectinate ctenidium (#18) and gonoduct independent of the kidney (#77) are atypical for Vetigastropoda among currently known members. However, for resolution of phylogenetic relationships, there are still many problems in the details of character states in this group. In previous studies, Quinn (1983) described the basic organization of Seguenzia, but his observations were limited to the macroscopic level. Haszprunar (1988 b) schematically showed the configuration of several pallial and coelomic organs in Flexinella, but did not show details. In this study, some features were observed by SEM but the histological details were unsatisfactory. Thus, many features still remain to be investigated. V-2. Evolution of Anatomical Characters1. Symmetry of Pallial OrgansCondition of the archaeogastropod pallial complex: The gastropod pallial cavity generally contains (i) true ctenidium (-a) and/or one of several types of secondary gill, (ii) osphradium (-a) probably derived from the dorsoterminal sense organ of primitive molluscan groups, (iii) hypobranchial gland (s) probably elaborated from the mucus tracts in the primitive pallial groove, and (iv) anal, excretory and genital openings (the latter two incorporated as a urogenital opening in most archaeogastropods). Very interestingly, "Archaeogastropoda" is unusual among Mollusca in that the pallial complex and its associated organs exhibit a mosaic pattern of symmetrically paired, asymmetrically paired, and unpaired conditions (Table 4). However, Caenogastropoda invariably exhibit unpaired monopectinate ctenidium, an unpaired osphradium, an unpaired hypobranchial gland, a monotocardian heart not traversed by the rectum, and an unpaired kidney.
The asymmetry of these organs in gastropods may be caused by differential growth between right and left sides as a coiling effect. However, causes and mutual relationships among animal asymmetry, shell coiling, and torsion are not very evident. At least the relationship between torsion and shell coiling is independent as exemplified by some patellogastropod limpets bearing truly symmetrical shells (see section V- 4). Conversely, symmetrical patellogastropods have asymmetrical organ arrangements except for paired osphradia. Only the fissurellid pallial cavity is seemingly completely bilateral as in non-gastropod molluscs, but condition is secondary as revealed by shell ontogeny (see section V-4). Possible secondary origin of the zeugobranch condition: Historical review shows that the primitiveness and irreversibility of the paired zeugobranch ctenidia and pallial complex have long been considered unquestioned dogma (Young, 1947; Fretter and Graham, 1962; Golikov and Starobogatov, 1975; Salvini-Plawen, 1980; Haszprunar, 1988 b; Ponder and Lindberg, 1996, 1997). Only Haszprunar (1990) considered the possibility of its secondary origin but original paired condition was still favored. The phylogeny reconstructed in this study shows that paired ctenidia are a logically reversed secondary state which occurs only in the zeugobranch clade independent of the outgroups (Fig. 106). This result may seem "unnatural" or "impossible" in a traditional view. However, assumptions favoring irreversibility in the gastropod ctenidium must postulate multiple independent changes from paired to unpaired states in many taxa throughout Gastropoda under constructed tree topology.
If the reversal is accepted, there are several lines of supporting evidence for the secondary, apomorphic state of zeugobranch ctenidia. (i) The number of molluscan ctenidia is variable and not very conservative as is typically found in non-gastropod groups. Often structurally homologous ctenidia are added or reduced at different sites. (ii) Ontogenetically the left ctenidium is initially predominant in contrast to the equally paired condition in adults (Crofts, 1937; Ino, 1952). Thus, ontogenetic criteria guarantee that the asymmetrical condition is original, not secondary. (iii) The presence of paired ctenidia is always reflected in apertural morphology of the shell in Recent members. The available fossil record shows that the earliest gastropod Aldanella had a slit-less trochoid shell (Runnegar and Pojeta, 1985), and pleurotomaroids with a slit appeared later. This suggests that zeugobranchs derived from coiled form with unpaired ctenidium. (iv) The ctenidia of vetigastropods exhibit not ancestral but rather derived states in the presence of skeletal rods, bursicles, and a long efferent membrane (Salvini-Plawen, 1980; Haszprunar, 1988 b). Thus, the osphradioctenidial complex (including their ganglion) could have arisen secondarily on the right side as a character complex. Patellogastropod "ctenidium": The scenario obtained in this analysis (Fig. 106) includes another confusing situation. Although the acmaeoid "ctenidium" was assumed to be homologous to other molluscan ctenidia in the character analysis, the reconstruction suggests that it was an independently acquired struc ture. However, this result seriously contradicts the theory of ctenidial homology about which general agreement has been established (Young, 1947; Fretter and Graham, 1962; Salvini-Plawen, 1980; Graham, 1985; Haszprunar, 1988 b; Ponder and Lindberg, 1997). It would be difficult to infer that such a complex structure was regained in an identical position with similar form, once it has been lost (Dollo's law). No clear solution for this problem can be presented from the results of this study. Patterns in other pallial organs: Under most-parsimonious reconstruction (fig. 104), transformations of other pallial organs can be traced and are summarized as follows. These results exhibit a general pattern from paired to unpaired conditions.
(1) Number of osphradia: The reconstruction resulted in the scenario that the osphradium was not present in early Gastropoda, because it is of questionable state in Nautilus and is absent in some Patellogastropoda. Paired osphradia arose on the pallial floor in some Patellogastropoda. In the Orthogastropoda, the left osphradium was retained (as in Neritopsina, Cocculina, Neomphalus, and Apogastropoda), and it was regained in paired condition in Vetigastropoda. (2) Number of hypobranchial glands: The hypobranchial gland is absent in Nautilus and in ancestral Gastropoda (presumably) plus Patellogastropoda (=Eogastropoda). The left gland arose in Orthogastropoda as is found in Cocculina, Seguenzia, Lepetodrilus, and Apogastropoda. The paired condition was re-acquired by common ancestor of Zeugobranchia and Trochoidea. The state having right gland only was evolved independently by Neritopsina and Anatoma. (3) Number of auricles: Two pairs of auricles (in the outgroups) were presumably reduced to the left one only in gastropod ancestor. This condition was inherited by Patellogastropoda and the orthogastropod ancestor (as in Neomphalus, Cocculina, Waldemaria, and Apogastropoda). The paired condition was independently re-evolved nearly symmetrically in Vetigastropoda and extremely asymmetrically in Neritoidea. (4) Number of kidneys: The symmetrically paired kidneys of the outgroups were converted into a single asymmetrical pair in Patellogastropoda and the left one in orthogastropod ancestor. Only Vetigastropoda evolved paired condition (and papillary sac) independently. These results show that all components of the pallial complex represent reversed, apomorphic conditions in zeugobranchs. This suggestion is "implausible" and may be difficult to accept in a traditional sense. Nev-ertheless, there is no theoretical reason why the unpaired condition in gastropod organs should be absolutely irreversible. These possibilities should be tested again in future works as should assumptions of homology problem. 2. Respiratory OrgansPolarity of ctenidial characters: Archaeogastropod ctenidia are characterized by the following features: (i) ctenidial leaflets or lamellae attached to ctenidial axes alternately on either side (bipectinate condition); (ii) each axis contains afferent and efferent ctenidial vessels and nerves; (iii) ctenidial axes are also provided with retractor muscles, and therefore the ctenidium is contractile when the skeletal rods are absent; (iv) triangular leaflets are stiffened by the skeletal rods in some but not in others; (v) each leaflet has three (frontal, abfrontal, and lateral) zones of cilia; (vi) the ctenidium is attached to the mantle skirt by efferent and afferent membranes; (vii) ctenidial sense organs (bursicles) may be present. Polarity of ctenidial characters were determined as follows by outgroup comparison (Salvini-Plawen, 1980; Haszprunar, 1988 b; Ponder and Lindberg, 1997): (i) the paired condition is primitive and a single left ctenidium is apomorphic; (ii) the bipectinate condition is plesiomorphic, and the monopectinate condition is apomorphic; (iii) presence of a long efferent membrane and of bursicles is apomorphic; (iv) presence of skeletal rods is apomorphic, while absence of skeletal rods (with ctenidial contractility) is plesiomorphic. Skeletal rods are an apomorphic character because of their absence in acuriferan ctenidia (Salvini-Plawen, 1980, Haszprunar, 1988 b) and are considered to be of independently origin in Bivalvia, Cephalopoda (uniquely in afferent axis), and Gastropoda (Young, 1947). In the reconstruction, the above-mentioned transformations are supported with two exceptions. (i) The paired condition may not be plesiomorphic as discussed above. (ii) The two equally supported transformations are possible regarding the presence of skeletal rods. Skeletal rods may evolved independently in nonneritopsine Rhipidoglossa and Caenogastropoda or lost by reversal in Neritopsina once obtained in the orthogastropod ancestor. Archaeogastropod monopectinate ctenidium: It is a general feature of prosobranch gastropods that the ctenidium is bipectinate in "Archaeogastropoda" but monopectinate in Caenogastropoda. However, there are some exceptions to this rule among archaeogastropods. A monopectinate condition is found in Lirulariinae and Umboniinae of Trochidae (Pretter, 1975; McLean, 1986; Hickman and McLean, 1990; Herbert, 1992), Larocheinae of Scissurellidae (Marshall, 1993 b), Anatominae of Scissurellidae (this study), Seguenziidae (Quinn, 1983; this study), and Skeneidae (Hickman and McLean, 1990; Warén, 1991 a). Such a ctenidial modification in trochoids is known to be correlated with suspension feeding with ctenidial cilia (McLean, 1986), but in most cases (except Umbonium) it also appears to be convergent related to small body size. Elongation of the ctenidial filaments is also the common convergent feature in various filterfeeding gastropods and also occurs in "Mesogastropoda" (e.g. Calyptraeidae and Vermetidae) (Declerck, 1995). These homoplastic phenomena between Vetigastropoda and Caenogastropoda are distinguished by the presence or absence of bursicles on ctenidial lamellae. Gills of Cocculiniformia: An unusual form of gill is markedly developed in "Cocculiniformia," and they can be divided into three types (Haszprunar, 1988 c). (i) Cocculinoidea (Cocculinidae and Bathysciadiidae) have a pseudoplicate gill whose position, innervation (from the osphradial ganglion), and blood supply are as for a true ctenidium. (ii) "Lower" Lepetelloidea (Lepetellidae, Osteopeltidae, Pyropeltidae, and Psudococculinidae) have several leaflets within the right subpallial and/or pallial cavities. The leaflets are provided with sensory pockets (except in Osteopeltidae). (iii) "Higher" Lepetelloidea (Addisoniidae and Choristellidae) have gills with a proximal glandular area and skeletal rods at the efferent axis. The question still remains whether the above gills are independent structures or modified ctenidia (Ponder and Lindberg, 1997), although Haszprunar (1988 b, c) regarded them as completely secondary. In the Cocculinoidea, homology with the ctenidium may be suggested by a lateral zone of cilia on the gill leaflets (McLean and Harasewych, 1995: fig. 19) in addition to similarity in position, innervation, and blood supply. In the Lepetelloidea, Ponder and Lindberg (1997) assumed secondary enlargement after reduction, because (i) skeletal rods are present in Addisoniidae and Choristellidae, and (ii) a bursicle-like pocket structure is found in Pyropeltidae, Lepetellidae, and Pseudococculinidae. Zoned ciliation on gill lamellae is also reported in Pseudococculinidae (Hasegawa, 1997 b: fig. 15). Gill reduction into a ciliary spot in Cocculinellidae may be correlated with extreme elongation of subpallial cavity. One of the difficulties in relating the lepetelloidean gill to a ctenidium is its position on the right side. However, it can be interpreted as a left organ based on the innervation from osphradial ganglion derived from left pleural ganglion (Ponder and Lindberg, 1997). Another problem is the paired condition of Caymanabyssiinae of Pseudococculinidae (Haszprunar, 1988 c, fig. 1). If the gill in Pseudococculinidae is a modified ctenidium, it follows that this represents independent conversion from unpaired to paired condition in this subfamily. Other types of vestigial gills: Aside from the gills of some Cocculiniformia, two other types of vestigial gills are known in archaeogastropods. (i) Nerita has a tuberculate expansion in a position similar to that of the true (right) ctenidium (Fretter, 1965: fig. 1 c; Fig. 77 c). However, this is not a general neritid character and therefore of minor significance to higher systematics of that family. At least Septalia do not have such a gill. (ii) Several leaflet-like processes are found in the right efferent pallial vessel of Lepetodrilus nux (Fig. 65 d; but undescribed in other lepetodrilids) and of Neomphalusfretterae (described but unillustrated, Fretter et al., 1981). Each of these may represent an intermediate state from azeugo-to zeugobranch organization, but the nature of these gills has not yet been fully elucidated. At least the lepetodrilid gill on the right side may be associated with the ctenidium because of its position and ciliation on the lamellae.
Patellogastropod secondary gills: Secondary gills in a circumpallial arrangement are developed in three patellogastropod groups, namely Patellidae, Nacellidae, and three genera of Lottiidae (Scurria, Tectura and Lottia) (Lindberg, 1988 a). This type of gill has have probably evolved several times, namely, once in the common ancestor of Patellidae and Nacellidae and at least three times within Lottiidae. In Patelloidea, the presence of this kind of gill seems to be a strictly phylogenetic phenomenon because all species have this structure. The difference in configuration of patellid and nacellid gills was probably caused by the autapomorphic change in the circulatory system of Nacellidae. In contrast, the presence of secondary gills in lottiid genera is considered to be an ecologically controlled phenomenon. The secondary gills are generated convergently by increased body size in Lottia gigantea (fide Lindberg, 1986 b, 1988 a), or by physiological stress in the tropical waters in Lottia, Tectura, and Scurria (Lindberg and McLean, 1981; Lindberg, 1988 a). Therefore, the presence or absence of such gills cannot be used as systematic character in the Lottiidae (Lindberg, 1988 a). Absence of the gill: In terrestrial neritopsines (Helicinoidea and Hydrocenoidea), the ctenidium is completely lost and replaced by the vascularized lung that is analogous to that of architaenioglossate and pulmonate land snails. Apparently, it is a common convergent pattern that terrestrial gastropods have also lost this distinct respiratory organ. In Patellogastropoda, neither ctenidium nor secondary gills are present in Lepetidae (Young, 1960; Angerer and Haszprunar, 1996) and Erginus (Golikov and Kussakin, 1972; this study). The absence of a distinct respiratory organ may be attributable to small body size and utilization of the pallial cavity as a blood pouch in the latter group. On the other hand, the absence of gills in Lepetidae is difficult to explain except as a result of phylogenetic constraint. Ecologically a correlation may be found with habitat on stones in muddy bottoms in cold waters of the subtidal zone to the deep-sea (Young, 1960). Body size seems to have no relationship with this phenomenon as exemplified by Limalepeta lima which attains more than 30 mm in shell length. 3. Buccal MassJaws: Archaeogastropod jaws are clearly differentiated into two distinct types (see section IV-1). Their evolutionary development can be traced as follows: (i) the number of jaws changed from primitively unpaired to dorsoventrally paired in Cephalopoda or bilaterally paired in Orthogastropoda; (ii) the cephalopod and gastropod ancestors shared the double-layered structure with muscular attachments to buccal mass by ACCTRAN optimization, but the similarity arose independently according to DELTRAN optimization. Thus, at least it is without doubt that the double-layered, undivided jaw fixed on the odontophore vs. single-layered, bilaterally divided jaws on the oral tube supports a major basic division of Gastropoda into Patellogastropoda and Orthogastropoda as proposed by Ponder and Lindberg (1996; 1997). Although it has not been conceived in previous comparative studies, the cephalopod upper beak and the patellogastropod jaw share some comparable states. As discussed in the character analysis section, they share a medianly fused single plate consisting of anterior and posterior sections, which are associated with the odontophore by muscle attachment, unlike those of other gastropod groups. Therefore, the anterior and posterior wings of the patellogastropod jaw can be assumed to be homologous with the outer and inner lamellae of the cephalopod upper beak, respectively. These characters possibly reflect the primitiveness of Patellogastropoda as retained through common ancestry of Gastropoda and Cephalopoda. Odontophoral cartilages: The transformation of this character has not been discussed in detail in previous phylogenetic studies of Gastropoda. The following changes are expected to have occurred in the five types of archaeogastropod odontophoral cartilages. (i) Ancestral Gastropoda had a single pair of cartilages. This state was inherited by the "vent taxa," Cocculinidae, Seguenziidae, Lepetodrilidae, and Caenogastropoda. (ii) The cartilages were split into two (anterior and posterior) pairs independently in "Zeugobranchia+Trochoidea," in Neritopsina, and in Patelloidea (Patella and Cellana). (iii) Two (anterior and anterolateral) pairs of cartilages are the basic patellogastropod pattern, from which more complicated cartilages have arisen in Patelloidea. It is difficult to establish the direct homology between two pairs in Patellogastropoda (anterior and anterolateral cartilages) and Polyplacophora-Tryblidiida (medial and lateral cartilages). (iv) Neritopsine uniquely evolved median pair of cartilages. Therefore, it is concluded that single pair as in some archaeogastropods and Caenogastropoda is original gastropod state, and the number of cartilages increased variously by secondary division in several lines of archaeogastropods. Common plan of archaeogastropod buccal musculature: Archaeogastropod buccal musculature consists of several common muscle elements (Table 5). They include: (i) lateral and ventral protractor muscles of the odontophore, (ii) anterior levator of the odontophore, (iii) two pairs (lateral and median) of protractors of the subradular membrane, (iv) the retractors of the subradular membrane and radular sac, and (v) ventral approximator of the odontophoral cartilages. These muscles are all present in Polyplacophora and Tryblidiida, and therefore, their presence is plesiomorphic in Gastropoda. The radular muscle (m. radula longus) connecting with the shell in Polyplacophora and Tryblidiida (Wingstrand, 1985) is absent at least in Cephalopoda and Gastropoda.
Buccal musculature of Neomphalus: The buccal mass of the enigmatic vent-taxon, Neomphalus, was described and illustrated by Fretter et al. (1981: figs. 3-5). According to their description, composition of the musculature in this genus is basically of archaeogastropod-type with the above-mentioned basic set of muscles, although pallial and coelomic organs exhibit "mesogastropod-like" states. Most importantly, the ventral side of the radular sac behind the buccal mass is connected to the floor of the body cavity by a "radular retractor muscle (rr)" (=posterior retractor muscle of radular sac in this study). The presence of this muscle suggests the relationship with Vetigastropoda. Evolution from archaeogastropod to caenogastropod buccal musculature: Comparative anatomy of the buccal musculature reveals several "trends" from archaeo- to caenogastropod levels of organization: (1) Reduction in muscle number: The number tends to decrease from Polyplacophora to Gastropoda, and also from "Archaeogastropoda" to Neogastropoda. (2) Reduction of extrinsic muscles: The muscles extending from the surface of the odontophore to the wall of the snout are reduced in number in Caenogastropoda. This change increases freedom of the buccal mass and plays an important role in the development of active carnivorous predation and proboscis formation. (3) Development of intrinsic muscles: In contrast to the reduction of extrinsic muscles, intrinsic muscles elaborate into a more compact muscular unit. In this respect, the buccal mass of Ampullariidae (Berthold, 1991: figs. 242-246) is closer to those of lower Neotaenioglossa such as Littorinidae (Fretter and Graham, 1962: fig. 14) rather than "Archaeogastropoda," supporting the advanced state of Architaenioglossa over "Archaeogastropoda." (4) Loss of levators and depressors: Unlike amphineurans and archaeogastropod groups, Caenogastropoda generally lack a set of levator and depressor muscles. (5) Development of retractors of the odontophore: In "Archaeogastropoda," the retraction of the odontophore is a simply passive returning movement by relaxation of the protractor muscles (Graham, 1973). However, a pair of retractors are developed on the ventrolateral sides of the buccal mass in Caenogastropoda except Ampullariidae (e.g. Fretter and Graham, 1962: fig. 14; Davis, 1967; Carriker, 1943) and in Heterobranchia (e.g. Hubendick, 1956; Hurst, 1965; Hembrow, 1973). Proboscis formation in "higher" caenogastropods: The above-mentioned changes are all explained by morphological changes in the entire feeding apparatus and in the mode of feeding.In grazers of the archaeogastropod level, the buccal mass is fixed in the snout, and feeding is regulated by orientation of the snout and cyclic movements of the radula over the odontophore. In Caenogastropoda, the simple snout is further elaborated into an extensile proboscis. The tube of the proboscis can be everted and prolonged to contact prey, and buccal mass moved longitudinally in the elongated proboscis. The development of retractors is essential to the control of movement of buccal mass in proboscidiferous, active predators. The evolution from an acrembolic to a narrowly elongated pleurembolic proboscis caused the elongation of cartilages and reduction of radular tooth number from "Mesogastropoda" to Neogastropoda (Graham, 1973). 4. RadulaBasic number of gastropod radular teeth: The importance of the number of radular teeth has long been emphasized in phylogenetic studies in Gastropoda. The rhipidoglossate radula was considered primitive in classic studies, probably because of the ancient image of zeugobranchs and the contrasting state in "higher" stenoglossate taxa, but the idea has not been supported in recent studies. The primitiveness of the patellogastropod radula is elucidated by outgroup comparison. In the outgroups, tooth number is 17 (8+1+8) in Polyplacophora, 11 (5+1+5) in Tryblidiida, and 5-7 in Scaphopoda (Salvini-Plawen, 1988). In Cephalopoda, each row of the radula consists of 13 elements (central+2 laterals+2 marginals+2 marginal plates) in Nautiloidea or 9 elements (central+2 laterals+1 marginal+1 marginal plate) in Coleoidea and Ammonoidea (Nixon, 1995; Tanabe and Fukuda, in press). Therefore, the tooth number of 5-13 in Patellogastropoda is closer to the outgroups than to the higher numbers in rhipidoglossate radulae (McLean, 1979; Wingstrand, 1985; Salvini-Plawen, 1988; Lindberg, 1988 a; McLean, 1990 b). Common characters among Polyplacophora, Tryblidiida, and Patellogastropoda: In addition to similarity in tooth number, these three groups share several common features in radular morphology: (i) functional type is stereoglossate; (ii) teeth are mineralized with magnetite (Salvini-Plawen, 1988; Okoshi, 1996); (iii) the central tooth is small or lost; (iv) the cusp of the inner marginals is plate-like and combshaped in some Polyplacophora (the fifth teeth) and in Tryblidiida (the fourth teeth). A similar state is present only in Lepetidae within Patellogastropoda (McLean, 1979; Wingstrand, 1985); (v) The basal plate is absent. This state is likewise found only in Lepetidae and Neolepetopsidae within Patellogastropoda. These shared states strongly support the basal position of Patellogastropoda in Gastropoda, and also the primitive status of Lepetidae within Patellogastropoda. Therefore, outgroup comparison in morphology again suggests that the hystrichoglossate radula of Pleurotomariidae is far from the archetype (see also Hickman, 1984c). Changes in archaeogastropod radulae: In "Archaeogastropoda," the following changes are revealed by character tracing on the given cladograms. (i) The flexoglossate condition evidently evolved in the common ancestor of Orthogastropoda. (ii) Concerning tooth mineralization, two different interpretations are possible. In ACCTRAN optimization, mineralization was lost once in the common ancestor of Gastropoda and Cephalopoda and re-acquired by reversal in Patellogastropoda. In DELTRAN optimization, mineralized teeth were inherited by ancestral gastropods, and later demineralized in Orthogastropoda. (iii) The presence of basal plates is an unquestionable synapomorphy of Patellogastropoda except Lepetidae and Neolepetopsidae. (iv) The central tooth was lost independently in Acmaeoidea (Acmaeidae and Lottiidae) and Cocculinidae. (v) The number of lateral teeth is mostly three pairs or less in the primitive state and is retained in Patellogastropoda. In Orthogastropoda, tooth number was reduced to a single pair in Caenogastropoda or increased to four to five pairs in rhipidoglossate taxa. Tooth reduction occurred in Seguenzia in convergence state with Caenogastropoda. (vi) The number of marginal teeth increased markedly in the ancestor of Rhipidoglossa. (vii) Plate-like or vestigial marginal teeth are retained in Patellogastropoda. A further apomorphic state was created by elongation of the shaft of the marginal teeth in the ancestor of Orthogastropoda. Specialization in rhipidoglossate radulae: As revealed by the reconstruction, the following four changes must have taken place in the course of evolution from the basic docoglossate type to the rhipido glossate condition: (i) loss of mineralization, (ii) increase in tooth number towards five pairs of laterals and toward many pairs of marginals, (iii) elongation of marginal shafts (manifest change into basal flexoglossate condition), and (iv) acquisition of marked articulation through the development of basal extension of the teeth. Due to a combination of the above characters, the radula of Neolepetopsidae was considered to represent an intermediate condition between docoglossate and rhipidoglossate radulae (McLean, 1990 b). However, other anatomical characters suggest the independence of this family from the rhipidoglossate type, therefore, this may represent a change in this state in Patellogastropoda rather than a gradual change. Several other specializations occur in some rhipidoglossate radulae as suggested by this analysis. (i) Enlargement of prominent outer lateral teeth evolved independently in Cocculina, some Zeugobranchia, and Neritopsina. (ii) Asymmetry in teeth row become prominent in a part of Zeugobranchia (Hickman, 1981). (iii) Independent specialization also generated lateromarginal plates in Fissurellidae and protolateromarginal plates in Trochoidea. Aberrant rhipidoglossate forms: A modified or completely transformed type of rhipidoglossate radula occurs in various archaeogastropod taxa. Some Seguenziidae with taenioglossate-like teeth have, therefore, been previously considered as "Mesogastropoda" (e.g. Golikov and Starobogatov, 1975). Highly specialized aberrant morphology is found in trochoid subfamilies of unknown affinities (Hickman and McLean, 1990) and in "higher" Lepetelloidea (e.g. Addisoniidae and Choristellidae, Hickman, 1983; McLean, 1985 a; Marshall, 1996). In such cases, investigations on buccal mass structure (cartilages and muscles) may be very informative in clarifying their systematic position as suggested above. 5. Alimentary Tract and GlandsSalivary glands: It is extremely difficult to generalize archaeogastropod salivary glands due to diversifi- cation into several subtypes. They variably open into the buccal cavity directly or through long ducts, and the form of glands is also greatly variable. (1) Patellogastropoda: Great diversification in both the glands and the ducts has occurred extensively within Patellogastropoda. (i) The glands are large and separated from the buccal cavity behind the buccal mass. They are connected by a pair of long salivary ducts in Cellana and most Lottiidae (Nipponacmea, Lottia, and Patelloida) (Sasaki and Okutani, 1993 a; this study; Sasaki, unpublished data). (ii) Erginus and Yayoiacmea have small glands with a pair of long ducts (Sasaki and Okutani, 1993 b; this study). (iii) The glands of Patella are unusual among Gastropoda in having two pairs of ducts from single pair of large glands. (iv) Within Patellogastropoda, Limalepeta, Pectinodonta, and Niveotectura share obliquely tubular glands on the buccal mass without ducts. Subdivision of these states were not used in the analysis because of complexity. (2) Gland-less groups: The reconstruction indicates that the loss of salivary glands occurred in ancestor of Rhipidoglossa. In some neritopsine genera, the absence of the glands is functionally compensated by the extensive lining of glandular epithelium of the buccal cavity and the sublingual glands (Fretter, 1965). In other groups lacking salivary glands (Cocculina, Neomphalus, and Lepetodrilus), specialization of sublingual glands does not occur. (3) Vetigastropoda: The glands of Zeugobranchia and Trochoidea are unique in having ramified lumen and longitudinally slit-like openings to buccal cavity in contrast to sack-like glands and small pore-like openings of others. The result of the analysis shows that these glands arose secondarily after the original glands were lost in basal Rhipidoglossa. Radular diverticulum: The presence of this character is plesiomorphic for all of archaeogastropods in- cluding all vent taxa. It is also present in Architaenioglossa (Ampullariidae), while it is lost in Neotaenioglossa and Neogastropoda (Fretter and Graham, 1962). This character evidently supports the basal position of Architaenioglossa and the derivation of the remaining "higher" gastropod groups within Caenogastropoda. Esophagus: The primitive archaeogastropod esophagus is generalized by the following features (Salvini-Plawen and Haszprunar, 1987; Salvini-Plawen, 1988; Fig. 98): (i) division into three portions, viz. anterior, mid-, and posterior esophagi; (ii) dorsoventral depression of the anterior esophagus; (iii) a ciliated dorsal food channel and a ventromedian ciliary tract between the dorsal and ventral folds; (iv) lateral pouches on the outer sides of the anterior esophagus; (v) an esophageal valve projecting over the radular diverticulum; and (v) a counterclockwise twist in the mid-esophagus as a consequence of torsion. Most "Archaeogastropoda" retain these primitive features, but the anterior esophagus is simplified in Caenogastropoda and greatly modified in Vetigastropoda. In esophageal characters, Vetigastropoda clearly exhibits an apomorphic state in the inner structure of the anterior and mid-esophagi (papillate glands covering enlarged esophageal pouches). Stomach: The generalized non-patellogastropod archaeogastropod stomach comprises a toothed gastric shield, a protostyle (= "food-mucus rod"), a ciliated sorting area, major and minor typhlosoles, and a gastric caecum. According to outgroup comparison, the patellogastropod stomach has lost the style sac region and gastric caecum completely, and as such is apomorphic. In this phylogenetic reconstruction, it is equally possible that the gastric caecum arose independently in Nautilus and Rhipidoglossa, or that it was lost separately in Patellogastropoda and Caenogastropoda. The latter case seems more likely, because similar (probably homologous) gastric structures are also found in bivalves (Owen, 1966; Purchon, 1977). "Cocculiniformia" have a specialized stomach which appears to be correlated with the unusual mode of feeding in the deep-sea environment (Haszprunar, 1988 c: fig. 3). In Caenogastropoda, gastric anatomy becomes more simplified in connection with carnivorous feeding (Fretter and Graham, 1962). These forms were most likely derived from the above-mentioned basic type. Gastric caecum: The presence of simple caecum is regarded as the primitive state in Conchifera because it is also found in Cephalopoda and lamellibranch Bivalvia (Salvini-Plawen, 1988). Therefore, the presence of a large spiral caecum or total loss of the caecum represent more advanced states. Vetigastropoda generally have a large coiled caecum, but it is absent in limpets (Fissurellidae, Broderipia, and Lepetodrilus). Especially in Trochoidea, members with a trochispiral shell always have a large caecum (e.g. Figs. 53 e, 56 b), whereas only limpet-shaped Broderipia lacks it (Fig. 57 b). This example strongly indicates that a large coiled caecum is markedly reduced in association with the limpet shape as suggested by Graham (1939, 1985). In the case of Neritopsina, both coiled and uncoiled forms have the simple short caecum.
Crystalline style: In Castropoda and Bivalvia the crystalline style is probably developed independently from the primitive protostyle (Salvini-Plawen, 1988). Typically, herbivorous and deposit- or suspension feeding "mesogastropods" elaborate the crystalline style, but such specialization does not occur in archaeogastropods (Declerck, 1995). Loops of the intestine: Generally the archaeogastropod intestine is long and forms a so-called anterior loop on the right posterior side of the buccal mass. In Caenogastropoda, the intestine becomes shortened, and the anterior loop is lost. Although configuration of the archaeogastropod gut is greatly diversified into complicated forms, they have probably originated in a common pattern phylogenetically and ontogenetically. Interestingly, gut coiling in juveniles of Patella (Smith, 1935: fig. 27) is quite similar to that in adults of vetigastropods (e.g. Figs. 33 a, 53 e, 64 a). Therefore, the gut folding twice to form a single anterior intestinal loop must reflect an original archaeogastropod coiling pattern. Within "Prosobranchia," the intestine may have evolved from a complex type to simple one, correlated with a change in feeding from herbivorous to carnivorous as in the case of the stomach (Fretter and Graham, 1962).
6. Reproductive SystemConnection of the gonoduct with the kidney: The intimate coordination of the excretory and reproductive systems in Patellogastropoda and Vetigastropoda has been regarded as primitive gastropod condition (Graham, 1985; Haszprunar, 1988 b). However, this connection is the apomorphic state in the mostparsimonious reconstruction obtained by this analysis. The connection of the gonoduct with the kidney occurred independently three times in Tryblidiida, Patellogastropoda, and Vetigastropoda. A similar case also is apparent regarding the supposed primitiveness of paired kidneys. Because the evolution of the gonoduct is probably correlated with the condition of the kidney, reconsideration of homology may be necessary for both the renal organ and the associated gonoduct at the ultrastructural level. Pallial gonoduct of Neritopsina: By contrasting the archaeogastropod condition with those of "higher" gastropods, the elaboration of the pallial gonoduct is regarded as an irrefutable major change in gastropod evolution. This change can be briefly summarized as follows. (1) Female organ: Females of "higher" gastropods developed apparatuses for reception and storage of sperm and as well as secretion of egg covering material (Fretter and Graham, 1962; Fretter, 1984 b). (i) The "bursa copulatrix" receives sperm at copulation, which are then passed to the "seminal receptacle," where they are stored in alignment with their heads buried in the epithelial wall. (ii) The proximal "albumen gland" and distal "capsule gland" of the pallial gonoduct serve for the formation of encapsulated eggs. (2) Male organ: In male organs, the following apomorphic states evolved. (i) A "seminal vesicle" may be formed for the storage of mature sperm. (ii) A "prostate gland" is developed on the pallial gonoduct. (iii) A penis is formed and connected with the gonopore via a ciliated sperm duct or groove. A functionally similar set of the above components is possessed by Neritopsina and various "higher" Gastropoda. However, the pallial gonoduct of Neritopsina can be distinguished from that of Caenogastropoda in some respects (Fretter, 1965): (i) it is wholly enclosed within the anterior pallial vein; (ii) there is no gonopericardial duct in the renal section of the gonoduct; (iii) the right hypobranchial gland is confluent with the pallial gonoduct. Based on these differences, the origin of the neritopsine pallial gonoduct is assumed to be independent. It is highly problematic that no clear criteria of homology are applicable to the gonoducts of entire groups of gastropods at higher levels (Ponder and Lindberg, 1997). Much of the information available for Neritopsina is actually based upon descriptions of Neritoidea which therefore may not always represent general neritopsine conditions. For example, crystals for reinforcement of the egg capsule is restricted only to some Neritidae. There seem to be greater differences between neritoidean and non-neritoidean groups than are currently recognized, and further investigation is needed on the extensive non-neritoidean groups. Modification in deep-sea taxa: Internal (or semi-internal) fertilization and brooding in the pallial cavity are common strategies in deep-sea archaeogastropods. Various accessory apparatuses are developed as independent structures. (1) Seminal receptacle in female: Development of a receptaculum seminis is the most common strategy in deep-sea taxa and has apparently evolved several times. It is found in Neomphalus (Fretter et al., 1981), Melanodrymia (Haszprunar, 1989 b), Cocculinidae and Bathysciadiidae (Haszprunar, 1987 a; this study), Addisoniidae and Choristellidae (Haszprunar, 1987 b, 1992), Seguenziidae (Haszprunar, 1988 b; this study), and Lepetodrilidae (Fretter, 1988; this study). (2) Copulatory organ in male: Deep-sea archaeogastropods often develop copulatory organ variously from cephalic, neck, and epipodial regions. In "Cocculiniformia," Seguenzia, Lepetodrilidae, and Neritoidea (including shallow-water taxa), the organ arises from the right side, whereas Neomphalus fretterae and its coiled relatives (Cyathermia and Lacunoides) (Fretter et al., 1981; Warén and Bouchet, 1989), and Gorgoleptidae (Fretter, 1988) all use the left cephalic tentacle or left oral lappet. In Trochoidea, the epipodial penis from the right side occurs in Skeneidae (Fretter and Graham, 1977; Warén, 1991 a) and Bathymargarites (Warén and Bouchet, 1989). Most of the above examples are functionally analogous, but morphologically and positionally not homologous. 7. Nervous SystemChanges from archaeogastropods to "higher" gastropods: It is well accepted that concentration of the nervous system is a major event in gastropod evolution (Fretter and Graham, 1962: 307-308; Haszprunar, 1988 b; Ponder and Lindberg, 1997). Especially, the prosobranch circumesophageal nerve ring must have been evolved through the change from a hypoathroid (adjacent pleural and pedal ganglia) to an epiathroid (adjacent pleural and cerebral ganglia) condition. By tracing this character on the phylogenetic reconstruction proposed here and by contrasting archaeo- and caenogastropods, the following process can be proposed for the evolution of the nervous system: (i) the shift of the cerebral and pleural ganglia toward the posterior dorsal side; (ii) the reduction in length of the cerebral commissure and formation of a compact nerve ring; (iii) the loss of the labial commissure; (iv) the development of zeugoneury to enhance innerva-lion of the well-developed left pallial organs; and (v) the development of pedal ganglia and the shift from pedal cords to pedal nerves. These phenomena are explained primarily by the shift of three major gangli-onic pairs (Fretter and Graham, 1962; Haszprunar, 1988 b) and are associated with proboscis formation in "higher" caenogastropod groups (Graham, 1973). It is clearly evident that the hypoathroid circumesophageal nerve ring with a streptoneurous visceral loop and scaraliform pedal cords represent the primitive gastropod nervous configuration of the common ancestor. In Gastropoda, this state is possessed by all archaeogastropods and Ampullariidae of Architaenioglossa. Therefore, retention of the hypoathroid condition clearly supports the primitive status of the latter group within the Caenogastropoda. Labial ganglia and commissure: The labial commissure is present in both Patellogastropoda, Neri-topsina and Architaenioglossa. Because it can be regarded as a homologue of the subcerebral commissure of amphineurans, its presence must indicate a primitive state, and loss of the commissure is clearly apomor-phic in non-neritopsine Rhipidoglossa and "higher" gastropods. In contrast, the development of labial ganglia is found only in Patellogastropoda, and therefore the presence of these ganglia is without doubt apo-morphic within Mollusca. Thus, the labial commissure is of early origin, whereas the presence of labial ganglia is highly innovative. Neritopsina-Architaenioglossa condition: Although the phylogenetic relationships as reflected by anatomical characters between Neritopsina and Ampullariidae have never been discussed in detail, these two taxa have a similar nervous system configuration (Figs. 101 c, d). The most prominent similarities are the one-sided origin of the visceral loop, the presence of a pleural commissure, the very thin supraesophageal-cerebral parts of the visceral loop, and a labial commissure without labial ganglia. Therefore, these character sets except plesiomorphic labial commissure may represent one synapomorphic state that links these two groups. However, these states are completely homoplastic according to the most-parsimonious reconstruction. They were probably created by the shift of subesophageal part of visceral loop to right side (Berthold, 1991) independently in these groups. A similar but somewhat deconcentrated configuration of the nervous system is also reported in the naticid-like Globularia fluctuata (fide Kase, 1990). This type of nervous system will be an important phylogenetic character in phylogenetic studies of "lower" Caenogastropoda. V-3. Phylogenetic Evaluation of Protoconch Characters1. Phylogenetic Characters of ProtoconchMorphotypes of archaeogastropod protoconchs: In terms of the number of whorls and symmetry, the protoconchs of "Archaeogastropoda" can be clearly classified into four types, each of which corresponds to a higher taxonomic category defined by anatomical characters (Fig. 107, Table 6).
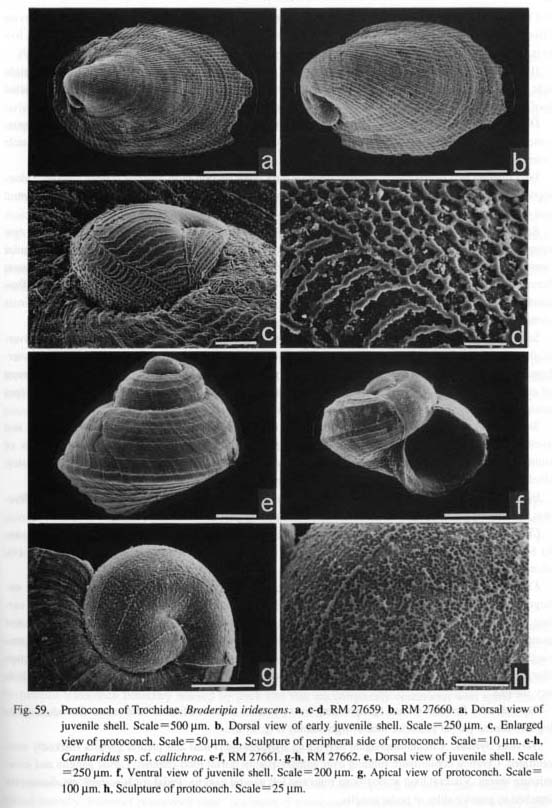
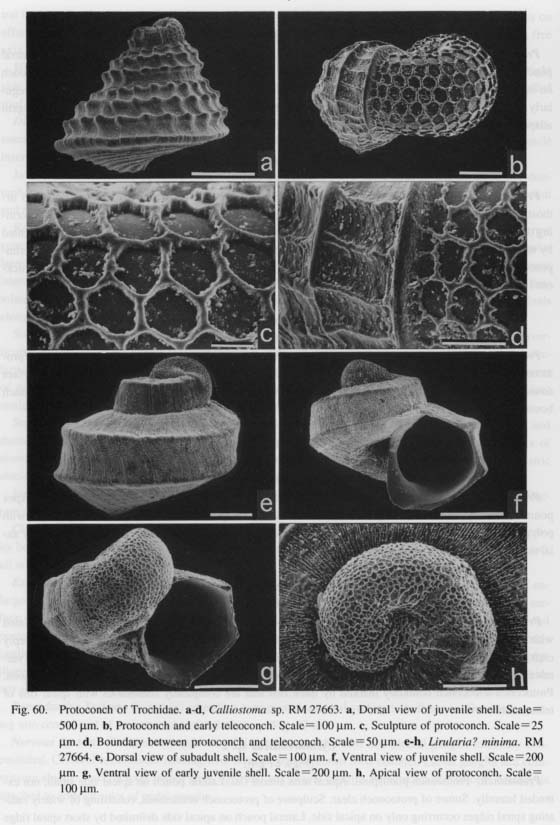
(1) Symmetrically uncoiled type: Truly symmetrical, bottle-shaped protoconchs are found in patellogas- ropod genera. The surface is generally covered with finely reticulate, wavy sculpture, although it is modified in the protoconch of brooded Erginus. The aperture of the protoconch is sealed by a septum after teleo-conch formation. (2) Paucispiral type: Vetigastropoda and their relatives (= non-neritopsine Rhipidoglossa) generally have a symmetrically or asymmetrically paucispiral protoconch. This type is further divided into four sub types: (i) a planispiral type in Cocculinoidea, (ii) a trochispiral type in Pleurotomariidae, Haliotidae, Crypeosectidae, Scissurellidae, Trochoidea, Seguenzioidea, Peltospiroidea, Neomphaloidea, and some of Fissurellidae (e.g. Rimula, Diodora, Tugali, and Scutus), (iii) an elongated type in some of Lepetelloidea (e. g. Psudococculinidae) and Fissurellidae (e.g. Emarginula, Zeidora, Puncturella, and Fissurella), and (iv) a symmetrical type in Macroschlsma of Fissurellidae (Fig. 40). It is common to all of these subtypes that the surface is covered with prismatic (typically net-like) deposits of various form.
(3) Multispiral type: Aquatic neritopsine groups have a multispiral protoconch regardless of the shell form of the teleoconch. Protoconch morphology is also unaffected by habitat differences as exemplified by various taxa from brackish (Septalia) to deep-sea (Shinkailepadidae) environments. This type differs markedly from the other in that the multispiral Protoconch II is formed by accretionary growth in the planktot-rophic veliger stage (Bandel, 1982). Resorption occurs in the inside of the protoconch. The surface is never adorned by prismatic deposits. (4) Globular type: Terrestrial neritopsines (e.g. Georissa of Hydrocenidae, Fig. 87) have a specialized dome-shaped protoconch. However, it is common to all neritopsine groups that the internal columella is re-absorbed and that the protoconch eventually becomes hollow. This characteristic of protoconch structure suggests derivation from aquatic forms due to secondary modifications to terrestrial life. Sculpture of protoconch: Within archaeogastropods, a sculpture specific to particular suprageneric taxa is found in only a few cases. Examples are restricted to thick transverse (axial) ribs in Scissurellinae of Scissurellidae, a hexagonal pattern in Calliostomatinae and Thysanodontinae of Trochidae, and a regular net-like pattern with a pitted surface in Cocculinidae. Therefore, in general, detailed differences in protoconch sculpture have little significance in higher-level systematics, but they are very useful in distinguishing closely related taxa at lower taxonomic levels such as a species-complex or genus. At the higher level, irregular prismatic (typically net-like) sculpture is not found in the protoconchs of Caenogastropoda (e.g. Bandel, 1975). Thus, such sculpture may be an important feature suggesting primitiveness retained in Patellogastropoda and non-neritopsine rhipidoglossate groups. Septal formation in Patellogastropoda: Formation of a septum and loss of the protoconch are common features in Patellogastropoda. The aperture of the protoconch is sealed by a septum from the inside, and ultimately the dorsal part of the protoconch is cut off usually at the stage of less than 1-2 mm in total shell, length. A small pit remains at the center of the septum after the absorption of the internal visceral hump (Morse, 1910; Smith, 1935: fig. 29). Also characteristically, a protoconch detachment scar in the shape of a figure-eight remains at the apex of the teleoconch (Sasaki and Okutani, 1993 a; Figs. 21 g, h). In other limpet-shaped gastropods, the protoconch is always retained at the apex except in fissurellids with foramen and some Lepetelloidea (e.g. Lepetella and Addisonia; Dantart and Luque, 1994). An internal septum is not known to occur in most non-patellogastropod groups, Pseudococculinidae produce a septum with an obliquely slit-like opening (Marshall, 1985 a: fig. 10 N; McLean and Harasewych, 1995: figs, 78, 82) but this exhibits a different mode of shell growth. Mechanical deformation in the paucispiral type: Bandel (1982) suggested that the asymmetrical shell in a paucispiral-type protoconch is fonned by a process called mechanical deformation. According to his interpretation, the protoconch is formed by the following successive events (see also Hickman, 1992). (i) A bilaterally symmetrical, cup-shaped organic shell is formed by the shell gland, (ii) The organic shell is mechanically modified into an asymmetrical form by the retractor muscle. Consequently, the apex is directed to the left, and the "lateral pouches" and corresponding dimple-like depressions are emphasized in the asymmetrical form. (iii) The organic shell is rapidly mineralized by the mantle following this deformation. Deformation prior to mineralization is supported by the presence of non-incremental lines mat are discordant to spiral sculpture (e.g. Fig. 59 g).
Heterostrophy in Trochoidea: In some genera of Trochoidea, the coiling axis of the protoconch is markedly tilted compared to that of the teleoconch, as is found in Lirular'ia? minima (Fig. 60 g) and Pondorbis japonicus (Fig. 61 b). Such a relationship is caused by the formation of a hyperstrophic protoconch and or-thostrophically coiled teleoconch. This change in the direction of shell coiling at metamorphosis is termed "heterostrophy" (Haszprunar, 1985 d; Bieler, 1993).
Heterostrophy is well-known as a synapomorphy of the Heterobranchia (Haszprunar, 1985 d). Apparent heterostrophy in Trochoidea differs from that in Heterobranchia in the following respects (Haszprunar, 1988 b; Bieler, 1992): (i) In Heterobranchia, the angle of heterostrophy ranges from 90 to 180 degrees (the extreme form is specially termed anastrophy or coaxial heterostrophy). In Trochoidea, the tilt of the protoconch axis is normally less than 45 degrees, (ii) The hyperstrophic state of the protoconch is formed by mechanical deformation prior to mineralization in Trochoidea, but by accretionary growth in Heterobranchia, as inferred from surface sculpture. Therefore, the similarity in these two distant groups represents an analo gous condition caused by different mechanisms of protoconch formation. Relationships between shell coiling and torsion: In the larval stage, the gastropod visceral mass is rotated counterclockwise by 180 degrees relative to the antero-posterior axis of the head-foot complex. This process is called torsion. The effects of torsion appear as asymmetry of rotation of the mid-esophagus, streptoneury of the visceral nerve loop, and spiral coiling of the larval operculum (Haszprunar, 1988 b). However, in shell morphology, some of patellogastropods (Lepetidae and Lottiidae) have a bilaterally symmetrical relationship between protoconch and teleoconch throughout ontogeny (Figs. 15, 21), although their soft parts are asymmetrically twisted. Therefore, it is clear that torsion of the visceral mass and asymmetry of protoconch and teleoconch are independent at least in the case of Patellogastropoda. Independence of torsion and shell coiling is also evidenced by the wide variety of coiling directions (from orthostrophic to hyperstrophic) in paucispiral protoconchs produced by similarly torted animals.
2. Correlations with Reproductive BiologyEffect of benthic vs. pelagic development on protoconch morphology: The mode of reproduction and spawning of archaeogastropods can be divided into several basic types (Table 7). Because the protoconch morphology is known to be affected by the ecological differences in reproductive and developmental strategies in Caenogastropoda (e.g. Lima and Lutz, 1990), it is important to discuss the relationships between morphotypes and ecological phenomena. In archaeogastropods, larvae are in most cases pelagic, but benthic development (= ecological definition of "direct development" by Thorson, 1950) has been reported in the following groups.
(1) Benthic egg mass: Larval development occurs within gelatinous egg masses in Margarites, Lirularia and Cantharidus (Amio, 1963; Hadfield and Strathmann, 1990; Ming and Sung, 1994). They deposit an egg mass, but its fertilization is still external. Larvae develop within the thick coating of the egg mass. Nevertheless, their protoconch morphology cannot be distinguished from those of species with pelagic development. These instances strongly indicate that developmental mode (pelagic or benthic) does not influence the morphology of the protoconch. (2) Brooding: Benthic development by brooding is known to occur among various archaeogastropods, especially in small-sized species of higher latitudes and of the deep-sea (Thorson, 1965; Hickman, 1992). Fertilization takes place within the pallial cavity, and larvae spend their entire developmental process there until they emerge at the crawling juvenile stage, (i) In Patellogastropoda, species of Erginus and Rho-dopetala (Lottiidae) are known to brood (Thorson, 1935; Golikov and Kussakin, 1972; Lindberg, 1979: this study). The protoconch of Erginus formed in the brooding pouch of the pallial cavity is greatly modified from the general patterns of other Patellogastropoda in form and sculpture (Figs. 72 a, b). In this case, the changes in form and sculpture may be attributable to brooding habit, because similar modification is not found in non-brooded patellogastropods. (ii) In Vetigastropoda, brooding is known in Larochea of Scis-surellidae (Marshall, 1993 b), Clanculus, Margarites, Arene, Muditia, and Tricolia of Trochoidea (Robert-son, 1985, table 7). However, these protoconchs do not differ dramatically in morphology compared to those of closely related, non-brooding species, (iii) In "Cocculiniformia," brooding is found in Cocculini-dae and Pseudococculinidae (Haszprunar, 1988 d), Bathyphytophilidae (Moskalev, 1978), and Lepetella (Waren, 1972; Dantart and Luque, 1994). Among these, the existence of brooding protection also cannot be detected from protoconch morphology.
According to the above examples, protoconch morphology is, in general, not affected by differences in life position during the larval stages. Thus, similarities in protoconch morphology can be assumed to reflect phylogenetic relationships in Archaeogastropods. Protoconchs I and II: In "higher" gastropods, it is well accepted that a clear demarcation of sculpture at the boundary between protoconch I (PI) and protoconch II (PII) reflects a difference between planktot-rophic and non-planktotrophic development (the so-called "apex theory") (Thorson, 1950; Shuto, 1974; Jablonski and Lutz, 1983; Lima and Lutz, 1990). Generalization for the relationship between protoconch features and reproductive strategies is summarized as follows (Hadfield and Strathmann, 1990; Hickman, 1992): (1) Species with planktotrophy have a small PI derived from a small egg. Their protoconchs have high spired multispiral whorls, on which an abrupt change of sculpture occurs at the PI/PII boundary. In Caenogastropoda, sinusigeral notch is often developed at apertural margin of PII (Bandel, 1975). (2) Species with non-planktotrophy (lecithotrophy, benthic development, and ovoviviparity) have a large protoconch (mostly PI) derived from a large egg, consisting of paucispiral whorls without distinctive sculpturing. Therefore, initial size, number of volutions, and presence or absence of the demarcation are used as criteria to distinguish planktotrophic and non-planktotrophic mode of development. In archaeogastropods, definite planktotrophic development has been verified only in aquatic neritopsines (Bandel, 1982; Robertson, 1985; Hickman, 1992). Non-planktotrophy is also anatomically indicated by the absence of the larval feeding organ, or metatroch (= post-oral ciliary band), and the ciliated food groove (Fretter and Graham, 1962; Hickman, 1992). Therefore, a dichotomy seems to hold between planktotrophic neritopsines and non-planktotrophic non-neritopsines within archaeogastropods. If this is true, the presence or absence of planktotrophy (and PII) is constrained phylogenetically in the case of archaeogastropods. Thus, the difference between multispiral (with PII) and paucispiral (without PII) protoconchs represents a phylogenetically essential character for archaeogastropods. 3. Evolution of Protoconch CharactersProtoconchs of outgroups: The formation of protoconch by shell gland is one of the manifest synapo-morphies of Conchifera (Wingstrand, 1985:51). It is therefore very important for the inference of ancestral state of gastropod protoconch to compare the protoconch morphology among conchiferan classes, (i) Try-blidiida has a circularly cap-shaped protoconch (Waren, 1988, 1992). (ii) Protoconch of Scaphopoda is elongated, tubular and longitudinally symmetrical (Engeser et al; 1993; Steiner, 1995). Right and left parts are distinguished by a longitudinal suture, (iii) Prodissoconch of Bivalvia greatly differs in paired condition from protoconch of other molluscs, but each of two valves is symmetrically shield-like (e.g. Waller, 1981). (iv) Nautilus has symmetrically cap-shaped embryonic shell. The central part of the surface is marked by depressions called "cicatrix" (Arnold et al; 1987; Tanabe and Uchiyama, 1997). Thus, the comparison among outgroups reveals that at least symmetrical protoconch is a general pattern of conchiferan molluscs except for most gastropods. Is protoconch asymmetry original or secondary?: Golikov and Starobogatov (1975) and Lindberg (1981 c, 1988 a) regarded that Patellogastropoda evolved from a conchologically coiled ancestor based on the asymmetrical growth pattern of the early teleoconch of Patella in which the protoconch is rotated by 20 degrees to the anterior-posterior axis of the teleoconch. SEM observations by Robson (1986) revealed that the somewhat sinistral appearance in the initial teleoconch of Patella (Smith, 1935: fig. 27) is caused by allometric growth of the apertural margin. This seemingly sinistral shell is, in fact, dextrally hyperstrophic shells produced by anatomically dextral animals. However, asymmetrical growth of the early teleoconch is not a general pattern in Patellogastropoda, but is very clear only in Patellidae (though the state of Nacelli-dae is unknown). The protoconch is almost symmetrically located in Lepetidae (Fig. 15), Lottiidae (Fig. 21), and Neolepetopsidae (McLean, 1990 b). This means that the coiled state in early teleoconch as in Patella is not necessarily regarded as original. Meanwhile, Haszprunar (1988 b) speculated that the gastropod archetype was a primarily symmetrical limpet ("primary limpet") like Patellogastropoda, so that helical coiling of most gastropod shells is a derived state. However, I regard that evolution of shell symmetry should be considered separately in regarding the protoconch and teleoconch. Concerning protoconch, outgroup comparison suggests the presence of a symmetrical protoconch in a gastropod ancestor independent of asymmetrically torted viscera, as is found in extant Patellogastropoda. On the other hand, variations in the degree of symmetry observed in early teleoconchs of Patellogastropoda mean that both coiled and uncoiled states were possible in the teleoconch of the gastropod ancestor. Thus, these facts indicate that a primitively symmetrical protoconch is likely, but the coiling direction of the ancestral teleoconch is not determinable. Evolutionary processes in archaeogastropod protoconchs: The taxonomic distribution of protoconch morphotypes (Fig. 107, Table 6) and inferred phylogeny (Fig. 104) suggest that the protoconch morphology of archaeogastropods are determined rather rigidly by phylogeny ---(i) symmetrically uncoiled type in Patellogastropoda clade, (ii) paucispiral type in "non-neritopsine Rhipidoglossa" clade, (iii) multispiral type in aquatic groups of Neritopsine clade, and (iv) globular type in terrestrial groups of Neritopsine clade. Among them only multispiral type of protoconch is produced by planktotrophic larvae.
Concerning the evolutionary pathways of protoconch characters, two ways of reconstruction are possible based on the inferred phylogenetic relationships: (i) Ancestral gastropods were non-planktotrophic, and bipartite protoconchs with planktotrophy evolved independently in Neritopsine and Caenogastropoda (at least two apomorphic changes). (ii) Ancestral gastropods were non-planktotrophic, and ancestral Orthogas-tropoda evolved planktotrophy from this. Non-planktotrophy was re-acquired in non-neritopsine Rhipidoglossa and some Caenogastropoda (single apomorphic change and more than two reversals). These opposing views are equally plausible. The former case becomes more likely, if emphasis is placed on sculptural similarities between protoconchs of patellogastropods and non-neritopsine Rhipidoglossa (as plesiomorphic) and on structural differences between neritopsine and caenogastropod protoconchs (as apomorphic). In this view, the following scenario can be presented for the evolution of protoconchs in "Ar-chaeogastropoda." (i) The symmetrically uncoiled protoconch of Patellogastropoda evolved from the symmetrically cap-shaped protoconch as in outgroups. (ii) Subsequently, protoconch morphology become pau-cispially coiled by asymmetrical mechanical deformation, as is observed in the "non-neritopsine Rhipidoglossa" clade. (iii) Finally, the paucispirally coiled protoconch was independently modified into the multispiral form in Neritopsina and in Caenogastropoda, as a result of accretionary shell growth of Protoconch II during the pelagic feeding veliger stage. Recently, the independent origin of planktotrophy between Neritopsina and Caenogastropoda has also been proposed by Haszprunar (1995) and Ponder and Lindberg (1997) based on different hypotheses on phylogeny. However, this conclusion presented here and by previous studies is still dependent on insufficient quantity and quality of data on archaeogastropod development. To test this hypothesis in more detail, more extensive observations are needed on larval morphology of various groups. At this point, evolution in gastropod protoconchs can be summarized by an initial change from symmetrical to asymmetrical form and a subsequent modification from paucispiral to multispiral form. |