IV. Phylogenetic Analysis
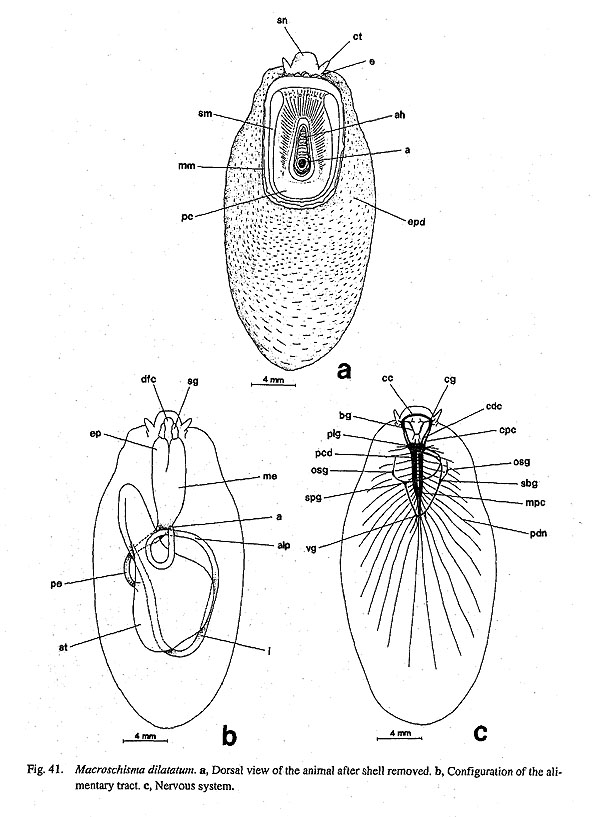
IV-1. Character StatesTo facilitate reference to state in OTUs, abbreviated generic names are listed after each character state. Abbreviations used in this section are as follows: Chiton = Chi, Neopilina=Nep, Nautilus = Nau, Patella =Pat, Cellana=Cel. Pectinodonta =Pec, Niveotectura = Niv, Nipponacmea=Nip, Erginus = Erg, Li-malepeta =Lim, Mikadolrochus =Mik, Sulculus=Sul, Scutus=Scu, Macroschisma=Mac, Anatoma= Ana, Turbo=Tur, Chlorostoma = Chl, Stomatia=Sto, Broderipia =Bro, Lepetodrilus = Lep, Seguenzia= Seg, Cocculina = Coc, Neomphalus=Nem, Nerita=Ner, Seplalia=Sep, Cinnalepeta = Cin, Waldemaria = Wal, Pomacea =Pom, and Biwamelania =Bw. 1. External Characters1. Mantle slit/hole: (0) absent [Chi. Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Tur, Chl, Sto, Bro, Lep, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) single [Mik, Sul, Scu, Mac, Ana], (2) double [Seg] The mantle margin of some archaeogastropods is deeply sinuate. In the zeugobranch genera (Pleurotom-ariidae, Haliotfdae, Scissurellidae, most Fissurellidae), there is a single slit between the paired pallial complexes as an exhalant sinus associated with the anal region. Members of Macroschisma have an apical hole instead of slit (Fig. 41 a), but it is functionally identical to the general zeugobranch condition. In Haliotidae, the shell has a series of pores but the mantle of the corresponding position has a simple slit. In Seguenzia, the mantle margin has two separated notches, viz. an inhalant sinus on the left side and an exhalant one on the right side. Their presence is also reflected in morphology of the shell aperture.
2. Retractile circmnpallial tentacles: (0) absent [Chi, Nep, Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Pat, Cel, Lim, Niv, Erg, Nip], (?) unknown [Pec] In patellogastropod genera, the mantle margin is fringed by numerous microtentacles. The length of mi-crotentacles is variable, from long in Cellana, to minute in Patella and Nipponacmea, to greatly diminutive in Niveotectura, Erginus, and Linalepeta. The microtentacles can almost completely be retracted into the inner fold of the mantle margin (Fretter, 1990), and therefore, they are often hardly visible and difficult to find in preserved specimens. Sensory projections are also elaborated on the mantle margins of members of various archaeogastropods, such as Mikadotrochus (Fig. 27 b), Lepetodrilus (Fig. 65 a), Cinnalepeta (Fig. 85 b), Seguezia (Fig. 67 a), and Anatoma (Fig. 43 c). However, they lack retractility unlike those of patellogastropod limpets.
3. Pallial tentacles: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv. Erg, Nip, Mik, Scu, Mac, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin. Wal, Pom, Biw], (1) present [Sul, Ana] In some zeugobranch gastropods, the margin of the mantle slit is provided with a few long tentacles, which are much more prominent than the circumpallial microtentacles. In Sulculus there are three tentacles, one in the posterior end of the slit and two on anterior sides situated asymmetrically (Fig. 29). In Anatoma, only a median tentacle is present (Fig. 42).
4. Snout: (0) absent [Chi, Nep, Nau], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg. Coc, Nem, Ner. Sep, Cin, Wal, Pom, Biw] It is a common character of Polyplacophora and Tryblidiida that the head region is less flexible with a muscular attachment to the shell or shell plate (Wingstrand, 1985). In contrast, Cephalopoda and Gas-tropoda have a free head that is liberated from dorsal shell covering. They differ, however, in the structure of the oral region. The mouth of Cephalopoda is deeply surrounded by a crown of arms/tentacles, whereas the archaeogastropod head is prolonged anteriorly as a snout so as to reach the substratum. 5. Cephalic tentacles: (0) absent [Chi, Nep. Nau], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] A pair of cephalic tentacles is always present in gastropods on the sides of the head; no non-gastropod mollusk has a homologous structure. In Archaeogastropoda the cephalic ganglia are always situated near the bases of these tentacles. 6. Position of cephalic eyes: (0) absent [Chi, Nep, Lep, Seg, Coc, Nem], (1) within lateral walls of head [Nau], (2) within bases of cephalic tentacles [Pat, Cel, Lim, Niv, Erg, Nip], (3) at posterior outside of cephalic tentacles (with or without eyestalks) [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Ner, Sep, Cin, Wal, Pom, Biw], (?) unknown [Pec] Several kinds of photoreceptors are often developed in various parts of the molluscan body. However, a pair of eyes innervated by the cerebral ganglia is shared only by Cephalopoda and Gastropoda. In other cases (e.g. pallial eyes of pectinid bivalves and valve eyes of Polyplacophora) receptors are innervated by pallial nerves from the pleural ganglia. The position of the eyes is slightly variable in several types. In Nautilus, they are invaginated in the lateral walls of the head (Young, 1987). In Patellogastropoda, they are housed within the bases of the cephalic tentacles. However, in members of non-patellogastropod genera, the eyes are always located on the outer bases of the tentacles, and are often provided with eyestalks of variable length. Eyes were not found in Lepetodrilus, Seguenzia, Cocculina, and Neomphalus (Fretter etal., 1981). A vestige of the original eyes has been identified as so-called "basitentacular glands" in Cocculinidae (Haszprunar, 1987 a). 7. Eyestalks: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Lep, Seg, Coc, Nem], (1) present [Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Ner, Sep, Cin, Wal, Pom, Biw] The outer bases of the cephalic tentacles may be evaginated to generate eyestalks and to carry the eyes at their distal tips. Eyestalks are not formed in Patellogastropoda but are present in members of rhipidoglos-sate and caenogastropod genera except in deep-sea eye-less taxa. Mikadotrochus lacks eyestalks even though eyes are present on the outer sides of the cephalic tentacles. The length of eyestalks is variable among the various gastropod groups. 8. Eye structure: (0) open, hollow [Nau, Pat, Cel, Lim, Niv, Erg, Nip], (1) open with vitreous body [Mik, Sul, Tur, Chl, Sto, Bro], (2) closed with vitreous body [Scu, Mac, Ana, Ner, Sep. Cin, Wal, Pom, Biw], (-) inapplicable [Chi, Nep, Lep, Seg, Coc, Nem], (?) unknown [Pec] Cephalic eyes are divided into several discrete grades of development. Among the outgroups. Nautilus has open pin-hole eyes (in contrast to lens eyes of other cephalopods) (Barber, 1987). Of Gastropoda, patellogastropod limpets have simply invaginated eyes, hollow internally, without contents of secreted materials (Fig. 92). The retina consists of a single layer of cells containing a pigmented zone (Marshall and Hodgson, 1990). In the remaining groups, the lumen of the optic cup is filled with a vitreous body (so-called "lens"). The pupil is covered with an epithelium in neritopsine genera and in Caenogastropoda but remains un closed in others.
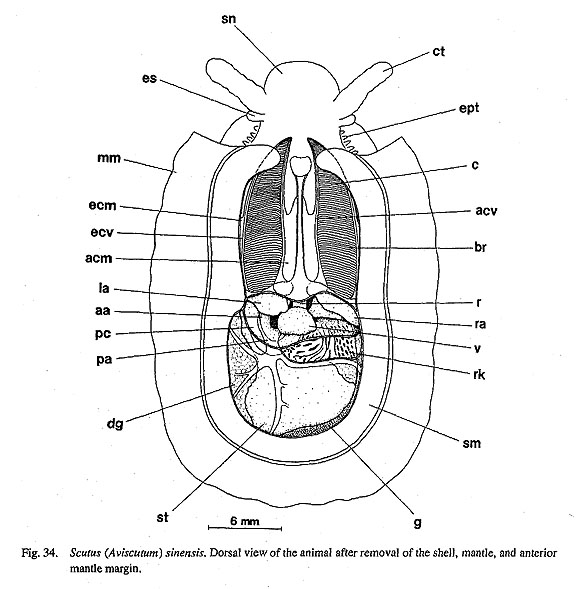
9. Cephalic lappets: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Scu, Mac, Ana, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Sul, Tur, Chl, Sto] A pair of flap-like extensions is present on the dorsal surface of the snout between the cephalic tentacles in Sulculus and in trochoidean genera excluding Broderipla. They are finely fringed and seem to be fused along the midline with each other and with the bases of the eye stalks on the outer sides. The cephalic penis in Neritidae may be comparable to this structure due to similarity in position (Ponder and Lindberg, 1997), but I do not treat them as homologous because of the asymmetrical condition of the neritoidean penis (character #82). 10. Neck lobes: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik. Sul, Scu, Mac, Anac Lep, Seg, Coc, Nem, Ner, Sep. Cin, Wal, Pomc Biw], (1) present [Tur, Chl, Sto, Bro] The epithelia of the right and left neck regions variously develop to form flap-like structures in the genera of Trochoidea. Such structures never occur in non-trochoidean gastropods and outgroups. They function as a guide for the inlet (left) and outlet (right) of water currents (Hickman and McLean, 1990). 11. Anterior pallial streaks: (0) absent [Chi, Nep, Nau, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc. Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Pat, Cel] Long strips termed "anterior pallial streaks" are situated at the anterior ends of the shell muscle in Patella and Cellana. Another pair of strips called "lateral pallial streaks" is absent in Cellana and restricted to Patella in patellogastropods (Pretter and Graham, 1962: fig. 50; Stüzel, 1984: pl. 21). They are said to be innervated from osphradial and pleural ganglia and to have sensory function (Haszprunar, 1985 a). Other groups of patellogastropod limpets and archaeogastropods lack structures homologous to these streaks. 12. Epipodial tentacles: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem] The surface of the epipodial region in archaeogastropods has various forms of tentacles that are innervated from the pedal ganglia (Crisp, 1981). Their morphology is greatly variable and difficult to categorize into discrete subtypes in terms of form, number, and position: (i) The most typical subtype is one with several pairs (mostly four symmetrical pairs) of long tentacles found in the trochoidean genera, Anatoma and Seguenzia. Neomphalus also has several pairs on posterior side, although numbers are different between right and left sides (McLean, 1981). (ii) Cocculina has only a single posterior pair of simple rod-like tentacles. (iii) In Mikadotrochus long tentacles are absent, but instead, several longitudinally elongated flaps are present (Fig. 24 a). Thick flaps similarly occur on the sides of the foot in Lepetodrilus. (iv) Scutus of Fis-surellidae has small triangular flaps along the epipodial margin (Fig. 34). However, in Macroschisma of the same family, vestigial tentacles are represented by tubercular processes on sides of the epipodium (Herbert, 1988). (v) In Sulculus, the tentacles are extremely hypertrophied to surround the entire epipodial region (Fig. 29). In the outgroups, cephalopod arm/tentacles may be comparable to gastropod epipodial tentacles, but their homologous relationship is rejected by the criteria of innervation (Salvini-Plawen, 1980; Salvini-Plawen and Steiner, 1996).
13. Epipodial sense organs (ESO): (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Mik, Sul. Scu, Tur, Chl, Sto, Bro, Lep, Seg], (?) unknown [Mac, Ana] The epipodial tentacles of many archaeogastropods are provided with special sensory structures at their bases. The epipodial sense organs are homologized by common position with the tentacles and by common innervation from the pedal nerves (Crisp, 1981), although the exterior form of the organ shows wide variation, from prominent knobs with cilia to simple tufts of cilia. These variations are divisible as follows: (i) The most typical form is knobby projections at the bases of the tentacles in trochoidean genera, (ii) Members of Fissurellidae have the sense organs on the ventral surface of each flap-like epipodial tentacles, although its presence or absence is unclear when the epipodial tentacles have degenerated. (iii) The presence of this organ in Pleurotomariidae has been questioned (Woodward, 1901; Fretter, 1964, 1966; Hickman, 1996), but tuberculate protrusions were observed with SEM during this study along the ventral margin of the epipodial flaps (Fig. 27 c). These are morphologically similar to the ESOs in Fissurellidae, and are hence considered to be homologous. (iv) Members of Haliotidae has a crown of cilia at the bases of epipodial tentacles. (v) In Scissurellidae, presence of sense organs is questionable. Similarly, Seguenzia has papillate epipodial tentacles but sensory organs could not be observed. The states in these taxa must still be considered dubious, because detailed observation was not successful during this study. For the analysis, the state in Seguenzia was scored as "present," following the data matrices of Ponder and Lindberg (1996, 1997). (vi) Lepetodrilus has tuberculate ESOs as projections on the epipodial flaps, (vii) Cocculina has a pair of posterior tentacles, but ESOs are lacking. None of Patellogastropoda, Neomphalus (fide McLean, 1981), Neritopsina, or Caenogastropoda possess similar sense organs in the epipodial region. 14. Micropapillae on cephalic and epipodial tentacles: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Scu, Mac, Lep, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Sul, Ana, Tur, Chl, Sto, Bro, Seg] The tentacles of some archaeogastropods are provided with papillae, each composed of a long stalk with a ciliary crown at the distal tip. They have been considered to function as sensory organs (Crisp, 1981) and termed "micropapillae" (Hickman and McLean, 1990). Micropapillae were found in Sulculus, all trochoidean genera, Anatoma (Fig. 43 d), and Seguenzia (Fig. 67 c). The absence of such microscopic structures was verified in other genera by SEM observation. The epipodial tentacles of Cocculina do not have micropapillae and are simply covered with cilia. 15. Number of shell muscle attachments: (0) eight pairs [Chi, Nep], (1) two pairs [Nau], (2) single pair [Sul, Lep, Ner, Wal], (3) unpaired (including horseshoe-shaped) [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Seg, Coc, Nem, Sep, Cin, Pom, Biw] Polyplacophora and Tryblidiida share the state of muscular structure consisting of eight longitudinally replicated pairs of three muscle sets (oblique, longitudinal, and transverse muscles) (Wingstrand, 1985). Nautilus has two distinctly separated pairs of head retractors (Griffin, 1900), although other cephalopods (Coleoidea) have a single pair of dorsoventral muscles consisting of head and funnel retractors (Wells, 1988). A single pallial adductor is also present in octopods but is absent in squids. Among Archaeogas-tropoda, muscle attachments are clearly separated only in Haliotidae, Lepetodrilus, some Neritidae (Nerita, but not Septalia), and Helicinidae. 16. Muscle constriction into bundles: (0) absent [Chi, Nep, Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Nem, Sep, Cin, Wal, Pom, Biw], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Coc, Ner] Division of each shell muscle into a continuous series of bundles occurs in all patellogastropod genera, Cocculina, and Nerita. In the former two taxa, blood vessels from the visceral haemocoel penetrate the shell muscle to reach the circumpallial vessel, which is clearly observable in living animals. The site of penetration corresponds to that of muscular constriction but is partly incongruent. In Nerita, the vessel penetration is poorly developed, but muscle division is very clear. Other neritopsine genera do not exhibit such a condition (e.g. Septalia). In view ofoutgroup comparison, the constricted muscles of archaeogastro-pods cannot be regarded as fused as in amphineuran muscles, because the latter are composed of distinctly paired sets that are longitudinally replicated, while gastropod muscle bundles do not have paired counterparts on both right and left sides. 2. Pallial Complex2-1.Ctenidia17. Number of ctenidia: (0) multiple pairs [Chi, Nep], (1) two pairs [Nau], (2) single pair [Mik, Sul, Scu, Mac, Ana], (3) left only [Pec, Niv, Nip, Tur, Chl, Sto, Bro, Lep, Seg, Nem, Ner, Sep, Cin, Pom, Biw], (4) absent [Pat, Cel, Lim, Erg, Coc, Wal ] It is generally accepted that the primitive molluscan ctenidium is a single symmetrical pair like that in Caudofoveata (Salvini-Plawen, 1988), to be secondarily multiplied or reduced in various taxa. All three outgroups have larger number of ctenidia than Gastropoda. They are numerous in Polyplacophora (Eernisse and Reynolds, 1994), 3-6 pairs in Tryblidiida (6 in Vema, 5 in Neopilina) (Haszprunar, 1997), and 2 pairs in Nautilus (Griffin, 1990). The ctenidia of Archaeogastropoda are categorized into three states: (i) paired, (ii) left ctenidium only, and (iii) absent or replaced by "secondary gills." The paired right and left ctenidia are present in Zeugo-branchia (=Pleurotomariidae, Haliotidae, Fissurellidae and Scissurellidae), but the remaining archaeogas-tropods as well as the Caenogastropoda have a single left ctenidium only. The ctenidium is absent in Li-malepeta, Erginus, and Waldemaria, in which the vascularized mantle skirt serves as the primary respiratory surface. In other cases the ctenidium is replaced by a secondary structure: in Patella and Cellana by leaflets in the pallial groove, in Cocculina by a pseudoplicate gill in the pallial cavity. 18. Ctenidial filaments: (0) bipectinate [Chi, Nep, Nau, Pec, Niv, Nip, Mik, Sul, Scu, Mac, Tur, Chl, Sto, Bro, Lep, Nem, Ner, Sep, Cin, Wal], (1) monopectinate [Ana, Seg, Pom, Biw], (-) inapplicable [Pat, Cel, Lim, Erg, Coc, Wal] Molluscan ctenidia generally have bipectinate structure (Fig. 93). Most archaeogastropods retain this original condition, but "higher" groups (e.g. all Caenogastropoda) are invariably monopectinate, namely, with the ctenidial axis completely attached to the mantle skirt and only ventral lamellae retained. However, monopectinate ctenidia are also found in Anatoma and Seguenzia (Fig. 67 d). Seemingly an intermediate condition is observed in Lepetodrilus whose ctenidial lamellae are monopectinate in the posterior two- thirds but bipectinate in the remaining anterior part. In this analysis this condition was scored as bipectinate because it is clearly retained as such in the anterior portion. Partial change into a posteriorly monopectinate condition also occurs in some trochoid groups (Hickman and McLean, 1990).
19. Skeletal rods on efferent side of ctenidial filaments: (0) absent [Chi, Nep, Nau, Pec, Niv, Nip, Ner, Sep, Cin], (1) present [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Nem, Pom, Biw], (-) inapplicable [Pat, Cel, Lim, Erg, Coc, Wal] Each leaflet in a molluscan ctenidium is often provided with a supporting tissue called a skeletal rod. In the outgroups, the rod is absent in both Polyplacophora (Salvini-Plawen, 1988) and Tryblidiida (Lemche , and Wingstrand, 1959; Haszprunar, 1997). The ctenidial leaflets of Cephalopoda (including Nautilus) have a similar supporting structure on the afferent side, not on the efferent side (Young, 1947). Thus, the cepha-lopod skeletal rod is not considered homologous to those of other molluscs but to be of independent origin (Haszprunar, 1988 b; Ponder and Lindberg, 1997). Among Gastropoda, the efferent rod is absent in both patellogastropod and neritopsine genera but present in the remaining taxa having ctenidia. 20. Brusicles (=ctenidial sense organs): (0) absent [Chi, Nep, Nau, Pec, Miv, Nip, Mik, Lep, Nem, Ner, Sep, Cin, Pom, Biw], (1) present [Sul, Scu, Mac. Ana, Tur, Chl, Sto, Bro, Seg], (-) inapplicable [Pat, Cel, Lim, Erg, Coc, Wal] Some archaeogastropod groups are known to have a special sense organ on the efferent side of each ctenidial lamella (Fig. 93). This organ, called a bursicle, was experimentally demonstrated to have a chemo-sensory function in Trochidae by Szal (1971). Later Haszprunar (1987 c) investigated the microstructure of this organ and also revealed its phylogenetic significance as a synapomorphy for Vetigastropoda. In this study I also confirmed (using SEM) that the taxonomic distribution of ctenidial sense organs is restricted to Vetigastropoda (including Seguenzia). Exceptionally within this group, bursicles were not found in Mika- dotrochus (Fig. 27 d), although Haszprunar (1987 c) reported their presence in Perotrochus of the same family, Pleurotomariidae. The homology of this organ with other 'bursicle-like' structures is easily demonstrated by comparing internal and external structures. A true bursicle has a ciliated groove that connects with the lateral cilia and ciliated lumen (Haszprunar, 1987 c; Fig. 49 d). Using this criterion, it is without doubt that Lepetodrilus lacks bursicles. A row of grooves are found on the ctenidial leaflets in Lepetodrilus (Fig. 65 c), but these never contain a ciliated pocket lumen. The groove on the leaflets presumably represents the depressed terminal ridge of the ctenidial lamellae due to unusual depression and modification of the ctenidial attachment in this case. Neomphalus also seems to lack bursicles (McLean, 1981: fig. 8). 2-2. Osphradia21. Number of osphradia: (0) paired [Chi, Pat, Cel, Nip, Mik, Sul, Scu, Mac], (1) single left [Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep. Cin, Pom, Biw], (2) absent [Nep, Lim, Pec, Niv, Erg, Wal], (?) unknown [Nau, Ana] In the outgroups, osphradia are represented by strips on the pallial roof on the posterior sides of the cteni-dia in Polyplacophora (Haszprunar, 1987 d), while they were unknown or absent in Tryblidiida (Lemche and Wingstrand, 1959; Haszprunar, 1997). In Nautilus, so-called "interbranchial papillae" may represent an osphradium, but its homology is uncertain (Ponder and Lindberg, 1997). In archaeogastropods, there are both paired and unpaired conditions, (i) Paired osphradia are developed in the patellogastropod limpets (Patella, Cellana, and Nipponacmea) and zeugobranchs (Pleurotomariidae, Haliotidae, and Fissurellidae). (ii) Only the left osphradium is developed in other gastropods. The osphradium of Neomphalus is also regarded as a single left one as discussed by Ponder and Lindberg (1997). The state in Seguenzia was also coded as single left, following Ponder and Lindberg's (1997) data matrix (character 100). (iii) The organ is absent in some patellogastropods (Limalepeta, Pectinodonta, Niveotectura, and Erginus) and Waldemaria (Helicinidae). The state is unknown in Anatoma. 22. Position of the osphradia: (0) on pallial roof [Chi, Pom, Biw], (1) on pallial floor [Pat, Cel, Nip], (2) on free portion of efferent ctenidial axis [Mik, Sul, Scu, Mac, Tur, Chl, Sto, Bro], (3) at base of efferent ctenidial membrane [Lep, Coc, Nem, Ner, Sep, Cin], (-) inapplicable [Nep, Lim, Pec, Niv, Erg, Wal], (?) unknown [Nau, Ana, Seg] The position of osphradia exhibits four states in outgroups and ingroups. (i) The osphradia of the Polyplacophora and Caenogastropoda are separated from the ctenidium and adhere to the pallial roof. (ii) In the patellogastropod genera, they are always on the floor of the pallial cavity just inside the anterior end of the shell muscle attachment, (iii) In the vetigastropod genera, they are on the efferent axis of the free tip of the bipectinate ctenidium. (iv) In Neritopsina, Lepetodrilus, Neomphalus, and Cocculina, the osphradium is associated with the base of the efferent side of the ctenidium. 23. Morphology of the osphradia: (0) single-zoned [Chi, Mik, Sul, Scu, Mac, Tur, Chl, Sto, Bro, Lep, Coc, Nem, Ner, Sep, Cin, Pom, Biw], (1) two-zoned with wart-organ [Pat, Cel, Nip], (-) inapplicable [Nep, Lim, Pec, Niv, Erg, Wal], (?) unknown [Nau, Ana, Seg] In archaeogastropods, two discrete states occur in the external morphology of the osphradium (Fig. 94). (i) In patellogastropod genera, osphradia consist of two distinct areas, viz. inner and outer zones ("Osphradium s. str." and "Ctenidienrudiment" of Stützel, 1984). According to Haszprunar (1985 a) the inner zone is directly innervated by the osphradial ganglia; its epithelium lacks sensory cells and is associated with free nerve endings only; the outer tuberculate zone (=wart-organ) includes numerous "cilia star cells." The wart-organ has sometimes been considered as gill rudiments (e.g. Stützel, 1984), but this idea is rejected because of the coexistence of wart-organ and ctenidium in some lottiid genera (e.g. Lottia and Nipponacmea). (ii) In the remaining groups of gastropods, osphradia lack such specific structures externally. In the outgroups, polyplacophoran osphradia consist of long vermiform strips without distinct zonation (Eernisse and Reynolds, 1994; Haszprunar, 1987 d).
24. Lateral ciliated zones: (0) absent [Chi, Pat, Cel, Nip, Mik, Sul, Scu, Mac, Tur, Chl, Sto, Bro, Lep, Coc, Nem], (1) present [Ner, Sep, Cin, Pom, Biw], (-) inapplicable [Nep, Lim, Pec, Niv, Erg, Wal], (?) unknown [Nau, Ana, Seg] Ciliated zones on sides of osphradium are found in neritopsine genera (except Waldemaria) and in caenogastropod genera, but are absent in other groups. In neritopsines and Biwamelania ciliated zones are parallel to elongated simple ridge of central zone of osphradium (Fig. 94 c). Pomacea has ciliated zones in grooves oflamellate osphradium. Polyplacophora lack the division of ciliated zones (Haszprunar, 1987 d). 2-3. Hypobranchial Glands25. Number ofhypobranchial glands: (0) paired [Chi, Nep, Mik, Sul, Tur, Chl, Sto, Bro], (1) single right [Ana, Ner, Sep, Wal], (2) single left [Lep, Seg, Coc, Pom, Biw], (3) absent [Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Scu, Mac, Nem, Cm] The molluscan pallial cavity is often provided with mucus-secreting glands. They are particularly well- developed as a prominent glandular area, the hypobranchial gland. In the outgroups, paired elongated strips are present in the pallial groove of Polyplacophora (Salvini-Plawen, 1988) and in Neopilina (Lemche and Wingstrand, 1959), whereas glands are unknown in Nautilus (Griffin, 1990). Within archaeogastropods, several states are distinguishable: (i) Paired glands are generally well-developed on either side of the rectum in most Vetigastropoda. In the dextrally coiling forms, the left gland is always larger than the right one that is suppressed on the columellar side. (ii) Most neritopsine genera are unique in having only the right gland which is attached dorsally to the gonoduct at the posterior end of the pallial cavity. The scissurellid Anatoma also has only the right gland (relative to the rectum) (Fig. 42 a). (iii) The mantle skirt completely lacks the glands in patellogastropods, Phenacolepadidae, Fissurellidae, and Neomphalus (McLean, 1981). (iv) All of the remaining archaeogastropods and caenogastropods examined have a single left hypobranchial gland. 3. Alimentary System3-1. Muscles of Oral Region26. Transverse labial muscles: (0) absent [Chi, Nep, Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip] In Patellogastropoda, the paired inner lips of the mouth are closely associated with the anterior wings of the jaw, and their movement is controlled by "transverse labial muscles" (Graham, 1964) that are unique to the group. In the remaining archaeogastropods and outgroups, the inner lips are free from the jaw plates, and this muscle is absent. 3-2. Muscles of Odontophore27. Dorsal protractor muscles of the odontophore: (0) absent [Chi, Nep, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin. Wal, Pom, Biw], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip], (?) unknown [Nau] The protractor muscles running between the posterior end of the cartilage and the wall of the snout on the dorsal side of odontophore is called the dorsal protractor muscle of the odontophore (Graham, 1964; 1973). This paired muscle is shared by all patellogastropod genera but is absent in all other groups including out-groups. The state in Nautilus is unknown in previous descriptions (Griffin, 1900; Young, 1991). 28. Anterior levator muscles of the odontophore: (0) present [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Nem, Ner, Sep, Cin, Wal], (1) absent [Coc, Pom, Biw] The levator muscles running from the anterolateral sides of the odontophore to the wall of the snout has been described in Polyplacophora and Tryblidiida (Graham, 1973; Wingstrand, 1985, as "Muscle IIIc=m. cartilaginis anterolateralis"). In Gastropoda, it is also present in all patellogastropod, vetigastropod, and neritopsine genera, but is absent in Cocculina and caenogastropod genera. A homologous muscle is also absent in Nautilus (Griffin, 1990). The "odontophoral levator" in Neomphalus (Fretter et al., 1981: figs. 3-4) is homologous with this muscle. 29. Dorsal levator muscles of the odontophore: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Pom, Biw], (1) present [Ner, Sep, Cin, Wal] This levator muscle originates from the anterodorsal wall of snout and inserts at the posteroventral end of the cartilage, pulling the odontophore anterodorsally. It is crossed transversely by the postdorsal buccal ten-sor muscle but for the most part lies on the dorsal surface of the buccal mass. Presence of this muscle is restricted to the neritopsine genera. 30. Median levator muscles of the odontophore: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Wal, Pom, Biw], (1) present [Ner, Sep, Cin] From the median sides near the dorsal levator muscles, the thinner muscle strands of the levator muscle are extended from the ventral end of the posterior cartilage to the dorsal wall of the snout. This muscle appears dorsally from the outside of the lateral pouch of the anterior esophagus and lies in the most dorsal position among all muscles. As in the former muscles, this occurs only in the neritopsine genera, but is absent in Waldemaria (Fig. 89 b).
31. Posterior depressor muscles of the odontophore: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Coc, Nem, Pom, Biw], (1) present [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro. Lep, Ner, Sep, Cin, Wal], (?) unknown [Seg] Some archaeogastropods have well-developed depressor muscles from the posterodorsal sides of the odontophore to the floor of the body cavity. It is present in vetigastropod and neritopsine genera but absent in the outgroups, patellogastropods, Cocculina, and Neomphalus (Fretter etal., 1981). An antagonistic levator muscle is, however, found only in some trochoid genera and Mikadotrochus. 32. Postdorsal buccal tensor muscle: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Nem, Pom, Biw], (1) present [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Coc, Ner, Sep, Cin, Wal], (?) unknown [Seg] The posterior dorsal part of the odontophore has a transverse tensor muscle to prevent the right and left cartilages from splitting apart (Fretter and Graham, 1962: fig. 108; Nisbet, 1973: fig. 6, as "postdorsal tensor of cartilages"). It always lies over the radular sac, the retractor muscles of esophageal valve, and the radular diverticulum. This muscle is present only in Vetigastropoda (including Lepetodrilus), and Neri-topsina. Neomphalus fretterae lacks this muscle (Fretter et al., 1981). 33. Dorsal buccal tensor muscles: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Coc], (?) unknown [Seg] This tensor muscle is extended obliquely from the posterodorsal surface of odontophore to the anterodorsal area over the buccal mass. The posterior part of this muscle is often attached to the postdorsal buccal tensor muscle, forming a tensor muscle complex. Presence of this muscle is restricted to Vetigastropoda (including Lepetodrilus), and Cocculina in this analysis. In most vetigastropod genera, the distal portion of this muscle is divided into two muscle bands; the upper band originates from the wall of the snout, while the lower band is inserted on the anterolateral extension of the anterior cartilages. The "net muscle" of coc-culinid Coccopigya (Haszprunar, 1987 a) represents a complex of dorsal and postdorsal buccal tensor muscles. 3-3.Jaws34. Number of the jaws: (0) absent [Chi], (1) single [Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip], (2) dorsov- entrally paired [Nau], (3) bilaterally paired [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] The presence of jaw(s) is a synapomorphy of Conchifera except bivalves lacking buccal mass (Wing-strand, 1985: 51), but their number and morphology vary greatly among them. Jaws are absent in Polypla-cophora (Wingstrand, 1985), single in Tryblidiida (Wingstrand, 1985), and dorsoventrally paired as "beaks" in Cephalopoda (Tanabe and Fukuda, in press). The gastropod jaw can be clearly distinguished as one of two types: an unpaired jaw in patellogastropods, or a paired jaw in non-patellogastropod groups, in-cluding Neomphalus (Fretter et al., 1981) (Fig. 95).
35. Structure of the jaws: (0) simple sheet-like [Nep, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) two-layered [Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip], (-) inapplicable [Chi] The cephalopods have dorsoventrally paired beaks with acute anterior tips as a primary feeding apparatus. Each of the upper and lower beaks consists of two layers: inner and outer lamellae (Tanabe and Fukuda, in press). In contrast, gastropod jaws always lie only on the dorsal side of the buccal mass. They are further divisible into two essentially different types: the patellogastropod jaw differs from that in other gastropods in having the form of a single plate with a double structure of anterior wings on the anterodosal sides and posterior wings on posteroventral sides. In the remaining groups, jaws consist of a pair of simple sheet-like plates. Tryblidiida also has a simple filmy jaw (Wingstrand, 1985). 36. Position of the jaws: (0) attached to the oral tube and free from the buccal mass [Nep, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) fixed on the odontophore by a muscle attachment [Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip], (-) inapplicable [Chi] Despite of great differences in jaw morphology (dorsoventrally paired or unpaired), cephalopod and patellogastropod jaws share a common structural feature. Cephalopod beaks are embedded in a membranous muscle sheet covering the buccal mass, and the inner and outer lamellae of the upper and lower beaks are attached to the buccal mass by thick muscles via a thin chitin-secreting cell layer (Dilly and Nixon, 1976). In the Patellogastropoda, a single jaw plate is also fixed on the dorsal side of the odontophore. The inner surface of the posterior wing has an attachment of muscles that are inserted on the anterolateral cartilages. In contrast to these groups, jaws are entirely attached to the oral tube independent of the buccal musculature in all of the other gastropod groups (Fig. 96) and Tryblidiida (Wingstrand, 1985).
37. Anterior edge of the jaws: (0) simple [Nep, Mik, Chl, Sto, Bro, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) calcified [Nau], (2) thickened into an anterior band [Pat, Cel, Lim, Pec, Niv, Erg, Nip], (3) fimbriate [Sul, Scu, Mac, Ana, Tur, Lep, Seg], (-) inapplicable [Chi] The anterior edges of the jaws of archaeogastropods and other molluscs exhibit several characteristic states: (i) The edges are simply smooth in Neopilina (Wingstrand, 1985), some of Trochidae (e.g. Chloros-toma. Fig. 53 c), Neomphalus (Fretter et al., 1981), Cocculina, Neritopsina, and Caenogastropoda. (ii) In Nautilus, the anterior tip of both beaks are heavily calcified (Okutani and Mikami, 1977; Saunders et al., 1978; Tanabe and Fukuda, in press). The upper beak has a pointed tip, and the corresponding margin of lower beak is denticulate, (iii) In the Patellogastropoda, the central part of the jaw covering the radular teeth is particularly thickened, and the anterior edge develops into a distinct zone called the anterior band (Walker, 1968). (iv) In most vetigastropods, including Lepetodrilus and Seguenzia, the edges are fimbriate with chitinous bristles or scaly projections.
3-4. Radular Sac38. Posterior end of the radular sac: (0) simple [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Ner, Sep, Cin, Wal, Pom, Biw], (1) bifurcated [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Coc, Nem], (?) unknown [Seg] Ponder and Lindberg (1996; 1997) introduced a new character of the radula that has been completely neglected in previous studies of gastropod phylogeny. It was also confirmed by this study that the end of radular sac is markedly bifurcated in some archaeogastropod groups, but it is quite simple in others. The former type is found in all vetigastropod genera (including Lepetodrilus, Fig. 64 b), Neomphalus (Fretter et al., 1981: fig. 3), and Cocculina (Fig. 70), while all remaining groups including the outgroups exhibit the latter condition. The states in Seguenzia was scored as "unknown" by Ponder and Lindberg (1997) and was also not revealed during this study.
3-5. Muscles of the Subradular Membrane and Radular Sac39. Retractor muscles of the subradular membrane: (0) divided into bilateral pair [Chi, Nep, Nau, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) fused ventrally [Pat, Cel] An identical topological position of the retractor muscle of the subradular membrane is shared by the cartilage-bearing molluscan groups. The subradular membrane is fixed on the odontophore by paired retractor muscles along the lateral margin. However, in Patella and Cellana, the muscles are extended ventrally to fuse at the midline (Graham, 1964: fig. 13) and are furthermore split into dorsal and ventral sections. It is also a common pattern in these two genera that the median and lateral protractors of the subradular membrane run between dorsal and ventral layers of the muscles (Fig. 5 d).
40. Symmetry of the median protractor muscles of the subradular membrane: (0) symmetrical [Chi, Nep, Nau, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) asymmetrical [Pat, Cel], (?) unknown [Seg] Patella and Cellana share a specialized configuration of this paired muscle, which greatly differs from the typical archaeogastropod pattern. In these genera, the left muscle runs almost straight into the body cavity behind the buccal mass; the right muscle bends abruptly to the right and further extends to the shell muscles (Graham, 1964: fig. 12; Figs. 5 c, d). This pattern does not occur in Polyplacophora, Tryblidiida, Nautilus, and other gastropod genera. Neomphalus is likewise reported to have symmetrical muscles as "protractor muscle of radular membrane" (Pretter et al; 1981: fig. 5,prm). 41. Postmedian retractor muscles of the radular sac: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pee, Niv, Erg, Nip, Ana, Lep, Coc, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Mik, Sul, Scu, Mac, Tur, Chl, Sto, Bro, New], (?) unknown [Seg] Characteristically paired retractor muscles are found in all vetigastropod genera except Anatoma. The fairly thick and paired muscle bundles originate from the floor of the body cavity behind the buccal mass. They are inserted on the ventral surface of the radular sac immediately posterior to the insertion of the retractor muscles of the radular sac. It is also a common pattern that the median protractor muscle of the subradular membrane ends near the origin of this muscle and that the median tensor of the radular sac is attached to the area near the insertion of this muscle. A homologous muscle in Neomphalus was described as "radular retractor muscle (rr)" by Fretter et al. (1981: figs. 3-5). It is absent in patellogastropod and neritop-sine genera, Anatoma (Fig. 43 f), Cocculina (Fig. 70), Lepetodrilus (Fig. 63 b), and Caenogastropoda. Its condition in Seguenzia is unknown.
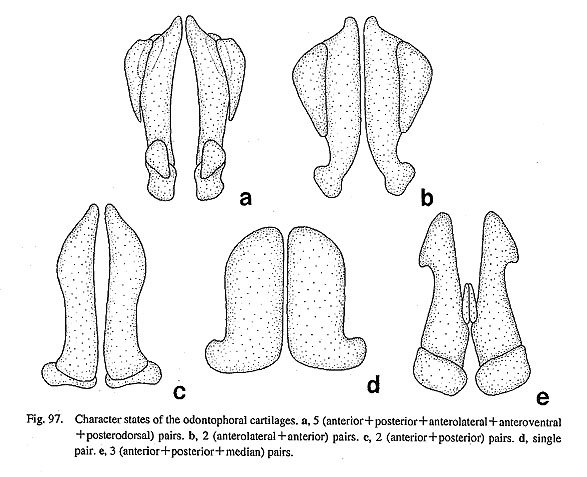
42. Median tensor muscle of the radular sac: (0) absent [Chi, Nep, Nau, Lim, Pec, Niv, Erg, Nip, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) inserted on the retractor muscle of the subradular membrane [Pat, Cel], (2) inserted on the sublingual pouch [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Coc], (?) unknown [Seg] A very thin but distinct muscle strand is inserted on the ventral surface of the radular sac in various archaeogastropod genera. Its origin is on the ventral side of the sublingual pouch in vetigastropod genera, Cocculina, and Lepetodrilus. In Patella and Cellana, a similar muscle is present, but its origin shifts slightly to the posterior margin of the fused ventral section of the retractor muscle of the subradular membrane (Fig. 5 d). Therefore, it may not be homologous but was tentatively identified here as this muscle because of the similarity in configuration. Other taxa, including Neomphalus (Fretter et al., 1981: fig. 5), lack a corresponding muscle. The state in Seguenzia is unknown. 43. Median retractor muscle of the radular sac: (0) absent [Chi, Nep, Nau, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Pat, Cel] Between the paired median protractor muscles of the subradular membrane, a thin retractor of the radular sac extends straight toward the posterior side in Patella and Cellana (Figs. 5 c, d). This characteristic muscle is shared only by these two genera, but is absent in all other groups. 3-6. Radular Teeth44. Functional type of radula: (0) stereoglossate [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip], (1) flexoglossate [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] In Gastropoda, the radula can be divided into two functional types that are referred to as "stereoglossate" and "flexoglossate" (Salvini-Plawen, 1988). The outgroups and patellogastropods have the former type, in which the teeth are moved only in parallel to the sagittal direction over the bending plane during feeding action. Gastropods other than patellogastropods all have the latter type radula in which the teeth exhibit flexible rotatory movement over the odontophore and are folded between paired cartilages when the radula is retracted. 45. Teeth mineralization: (0) mineralized [Chi, Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip], (1) non-mineralized [Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] In the outgroups, the polyplacophoran and tryblidiid radulae are darkly mineralized with iron (McLean, 1979; Wingstrand, 1985), but that of Nautilus is chitinous (Lowenstam et al., 1984; Tanabe and Fukuda, in press). In Archaeogastropoda, tooth mineralization with iron occurs only in patellogastropod genera (except Neolepetopsidae; McLean, 1990 b); all other gastropods have non-mineralized teeth. Ponder and Lindberg (1997) excluded this character from their phylogenetic analysis, because of uncertainty in homology concerning mineral contents. However, the recent results of chemical analysis show that the mineral composition is significantly different between dark (mineralized) and opaque (non-mineralized) teeth (Okoshi, 1996). Mineralized docoglossate radulae with a dark hue contain more than 1000 times more iron than non-mineralized flexoglossate teeth (Okoshi, 1996: table 1). Thus, a distinction between dark and opaque teeth is consistent with the result of chemical analysis, and the difference in color is useful to indicate the presence or absence of intensive mineralization with iron. 46. Basal plates: (0) absent [Chi, Nep, Nau, Lim, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Pat, Cel, Pec, Niv, Erg, Nip] Patellogastropod radular teeth (lateral teeth) generally rest on the specially thickened basal plates. They are morphologically complex in form and sculpture in Patella and Cellana, or represented by solid simple plates in Acmaeidae and Lottiidae (Lindberg, 1981 a, 1988 a; Lindberg and McLean, 1981; Sasaki and Okutani, 1993 a, b, 1994 a, b). Basal plates are totally absent in Lepetidae (Moskalev, 1977), all outgroups, and all remaining gastropods. 47. Symmetry of tooth row: (0) symmetrical [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) asymmetrical [Mik, Sul, Scu, Mac] The alignment of the right and left portions of a tooth row is generally symmetrical in the molluscan radula. In some rhipidoglossate radulae, however, asymmetrical arrangement of teeth is prominently developed, in Pleurotomariidae, Haliotidae, Fissurellidae (Hickman, 1981; 1984 a, c). In other taxa, the radula does not show clear asymmetry. 48. Central tooth: (0) present [Chi, Nep, Nau, Pat, Lim, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Nem, Ner, Sep. Cin, Wal, Pom, Biw], (1) absent [Cel, Pec, Niv, Erg, Nip, Coc] The radula of Testaria (Polyplacophora+Conchifera) normally has a medianly situated tooth termed central or rachidian tooth. However, there are some exceptional groups lacking it in archaeogastropods. (i) Acmaeid and lottiid genera and nacellid Cellana completely lack any trace of the central tooth. (ii) Coccu-lina also lacks the central tooth, and the radular membrane is widely exposed in the spacious area in the center. Among Patellogastropoda, Patella's central tooth is represented by a vestigial chitinous ridge between the basal plates (Sasaki et al, 1994). In Lepetidae, there is a large plate-like central tooth that is presumed to be fused with several pairs of lateral teeth (Moskalev, 1977). 49. Number of lateral teeth: (0) 3 pairs (including basal fusion) [Nep, Pat, Cel, Pec, Niv, Erg, Nip], (1) 4 pairs [Coc, Nem, Ner, Sep, Cin, Wal], (2) 5 pairs [Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep], (3) multiple pairs [Mik], (4) single pair [Seg, Pom, Biw], (5) absent [Lim], (-) inapplicable [Chi, Nau] The number of lateral teeth is variable in archaeogastropods and outgroups. (i) Patellogastropod genera normally have three pairs of lateral teeth (Lindberg, 1981 b, 1988 a; McLean, 1990 b), but often these teeth are fused at their bases. The apparent single pair of lateral teeth in Acmaeoidea can be regarded as three pairs because each tooth has three cusps which are still distinctly separated as in Pectinodonta (Tsuchida and Ishida, 1977; Hickman, 1983; Marshall, 1985 b). The lateral teeth of Tryblidiida is also regarded as three pairs (McLean, 1979). (ii) The number of lateral tooth pairs is four in Cocculina, Neomphalus (McLean, 1981), and neritopsine genera. (iii) There are uniformly five pairs in the Haliotidae, Pissurellidae, Scissurellidae, Trochoidea, and Lepetodrilidae. (iv) Mikadotrochus has markedly multiplied lateral teeth (ca. 40 pairs). (v) Seguenzia and Caenogastropoda have only a single pair. (vi) The lateral teeth of Lepetidae are probably involved in fusion, but its relationship with a plate-like central tooth is unknown. (vii) In the Polyplacophora and Nautilus, lateral and marginal teeth are nominally discriminated for description, but they are not sharply differentiated morphologically unlike the inner lateral and outer marginal tooth fields of Gastropoda. 50. Outer lateral teeth: (0) not differentiated [Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Tur, Chl, Sto, Bro, Lep, Seg, Nem, Pom, Biw], (1) fourth teeth enlarged [Coc, Ner, Sep, Cin, Wal], (2) fifth teeth enlarged [Scu, Mac, Ana], (-) inapplicable [Chi] One pair of outermost lateral teeth can be especially enlarged and differentiated. In fissurellid genera and Anatoma, the fifth teeth are much more prominent than other lateral teeth. A similar condition is found in the fourth teeth in Cocculina and neritopsine genera. Other groups of Archaeogastropoda, Cephalopoda, and Tryblidiida lack such specialization. The second lateral teeth of Polyplacophora are also enlarged, but their homology is uncertain. 51. Lateromarginal plates: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Ana, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) true plate [Scu, Mac], (2) basal extension of innermost marginal tooth (protolateromarginal plate) [Tur, Chl, Sto, Bro] The rhipidoglossate radula can have plate-like teeth that interact with outermost lateral and innermost marginal teeth at their base. Fissurellidae all have independent plates, but in Trochoidea they are in most cases continuous basally with the innermost marginal teeth. The simple plate of the latter form is considered to have arisen from the basal extension of the marginal teeth and is called "protolateromarginal plate" (Hickman and McLean, 1990; Hickman, 1996). Their homology can be difficult to definitely establish but they are treated here as such based upon similarity in morphology and position. The complete plate in Mar-garitinae of Trochidae (Hickman and McLean, 1990: fig. 50) may support this assumption. 52. Number of marginal teeth: (0) 3 or fewer pairs [Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Pom, Biw], (1) multiple pairs (normally more than 20 pairs) [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal], (-) inapplicable [Chi, Nau] It is very characteristic that non-patellogastropod archaeogastropods have many teeth of similar form in the marginal tooth field. In contrast, patellogastropods and the outgroups possess no more than three pairs of marginal teeth. Similarly, caenogastropod genera (Pomacea and Biwamelania) have only two pairs of marginal teeth. Tooth homology is uncertain for Polyplacophora and Nautilus in which comparable differentiation is not found in the outer teeth field. 53. Morphology of the marginal teeth: (0) plate-like [Nep, Pat, Cel, Lim], (1) inconspicuous or absent [Pec, Niv, Erg, Nip], (2) elongated with long shaft [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (-) inapplicable [Chi, Nau] The marginal teeth in archaeogastropods and the outgroups are divided into three states on the basis of the morphology of their shaft. (i) Tryblidiida, Patellidae, Nacellidae, and Lepetidae of Patellogastropoda share teeth with broad, plate-like cusp (Wingstrand, 1985). In these groups, each tooth is tightly attached to the radular membrane, (ii) The marginal teeth are greatly inconspicuous or completely absent in acmaeid and lottiid genera. (iii) The remaining archaeogastropod and two caenogastropod genera have teeth with a long shaft, small base, and slender cusp. 3-7. Odontophoral Cartilages54. Posterior cartilages: (0) absent [Chi, Nep, Nau, Lim, Pec, Niv, Erg, Nip, Lep, Seg, Coc, Nem, Pom, Biw], (1) present [Pat, Cel, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Ner, Sep, Cin, Wal] The possession of paired odontophoral cartilages in buccal mass is one of possible synapomorphies of Testaria (Polyplacophora+Conchifera), although it is absent in Bivalvia and Cephalopoda (Young, 1991). Among them, gastropods especially exhibit a diversity in the number of cartilages (Fig, 97). (i) Cartilages include anterior and posterior pairs in Patelloidea (Patella and Cellana), most vetigastropods, and neritop-sines. (ii) Most simple type is found in Lepetodrilus, Seguenzia, Cocculina, Neomphalus (Fretter et al., 1981), and caenogastropods in which only a single pair is present. Patellogastropods excluding Patelloidea also do not have posterior cartilages. In outgroups, Polyplacophora and Tryblidiida have two pairs ("medial" and "lateral") of cartilages and a single pair of fluid-filled vesicles (Wingstrand, 1985). Among these pairs, medial cartilages are regarded as homologous with anterior cartilages of gastropods based on similar insertion of ventral approximator (=muscle IIIa, m. radulae impar, Wingstrand, 1985: fig. 16). The homology of the remaining pairs with cartilages of gastropods is difficult to establish, although some possibilities have been proposed (Wingstrand, 1985: 61-64).
55. Anterolateral cartilages: (0) absent [Chi, Nep, Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip] Odontophoral cartilages of lepetid, acmaeid, and lottiid genera consist of two pairs (anterior+antero-lateral, not anterior+posterior). Cartilages of Patella and Cellana also exhibit a similar state in the antero-lateral pair, although these genera are specialized in total number of cartilages (three or five pairs). Therefore, odontophoral cartilages of all patellogastropods clearly differ from those of other molluscs in having anterolateral cartilages. 56. Median cartilages: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Pom, Biw], (1) present [Ner, Sep, Cin, Wal] All neritopsines genera investigated have a fused or unfused pair of median cartilages between anterior cartilages, and other gastropod genera totally lack this characteristic pair. "Median cartilages" in neritopsines are evidently different from "medial cartilages" described in Polyplacophora and Tryblidiida (Wing-strand, 1985), because the former are not inserted by the ventral approximator muscle. Thus, no other mollusks possess homologous cartilages. 3-8. Muscles of the Odontophoral Cartilages57. Ventral approximator muscle of the odontophoral cartilages: (0) single-layered approximator [Chi, Nep, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) two-layered approximator [Pat, Cel, Lim, Pec, Niv, Erg, Nip], (-) inapplicable [Nau] The inner sides of the anterior cartilages (Gastropoda) or the "medial cartilages" (Polyplacophora and Tryblidiida) are connected by a horizontal sheet of muscle (Graham, 1973; Wingstrand, 1985). This is always present in molluscan groups having paired cartilages. In the patellogastropod genera, the muscle is composed of ventral and dorsal layers; the ventral layer is fused horizontally below the cartilages but the dorsal one is inserted medially between the cartilages (Graham, 1964: fig. 13). Such a condition is not found in the approximator of the outgroups (e.g. Wingstrand, 1985: fig. 16, Muscle IIIa) and all of nonpatellogastropod gastropods, including Neomphalus (Fretter et al, 1981: fig. 3). 58. Outer approximator muscles of the odontophoral cartilages: (0) absent [Chi, Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Nem, Pom, Biw], (1) present [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Coc, Ner, Sep. Cin, Wal], (-) inapplicable [Nau], (?) unknown [Seg] In vetigastropod genera (including Lepetodrilus), Cocculina, and all neritopsine genera, thick bundles of longitudinal muscles connect the outer ventral sides of the anterior and posterior pairs of odontophoral cartilages or the anterior and posterior sides of a single pair of cartilages. This muscle is absent in Patellogastropoda, Neomphalus (Pretter et al., 1981: fig. 5), Caenogastropoda, and the outgroups. The state in Seguenzia is unknown. 59. Tensor muscles of the anterior cartilages: (0) absent [Chi, Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Pom, Biw], (1) present [Ner, Sep, Cin, Wal], (-) inapplicable [Nau] In neritopsine genera, a pair of thin muscles insert in the small area of anterior cartilage behind the anterolateral extension of the anterior cartilages. The topological relationship with other muscles exhibits a common pattern; i.e. this tensor emerges between the strands of lateral protractor muscle and extends to the origin in the inner wall of snout. No other molluscan genera have this muscle. 3-9. Alimentary Tract and Glands60. Outgrowths of sublingual gland: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Wal, Pom, Biw], (1) present [Ner, Sep, Cin] The sublingual pouch between the floor of the snout and the anteroventral side of the buccal mass (Fig. 96) has particular bilateral outgrowths in some groups. Species of Nerita have a pair of glandular pouches (Fretter, 1965: fig. 8; Figs. 74 c, 75 a). Among neritopsine genera, Septalia (Neritidae) and Cinnalepeta (Phenacolepadidae) also possess these pouches, but they are not present in Waldemaria (Helicinidae). Functionally, the absence of the salivary glands is compensated by the development of this gland in Neritidae (Fretter, 1965). However, in Cocculina japonica and Lepetodrilus nux, similar outgrowths are not found, although distinct salivary glands are absent. Of the patellogastropods, only Pectinodonta orientalis has pouches in a similar position (Fig. 16 d). However, very thin triangular sacs in this species are greatly different in appearance from round glandular sacs of Neritoidea. These structures are not regarded as homologous with the glandular pouches of neritopsines in this analysis. The functional significance of the triangular pouch of Pectinodonta is unknown, but it may be concerned with feeding on submerged logs in deep water.
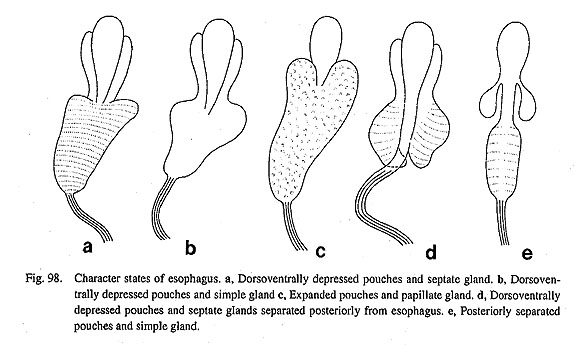
61. Salivary glands: (0) simple, sack-like glands [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Pom, Biw], (1) ramified glands [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro], (2) absent [Lep, Coc, Nem, Ner, Sep, Cin, Wal], (?) unknown [Seg] The salivary glands of Gastropoda and other molluscs are greatly variable in form and size. Although the glands of outgroups and patellogastropods can be split into a number of subtypes, they are divided into the following three states in this analysis. (i) In outgroups salivary glands are simple and sac-like without ducts in Polyplacophora (Fretter, 1937), Tryblidiida (Wingstrand, 1985), and Nautilus (Griffin, 1990: fig. 70). Patellogastropod glands are greatly diversified in terms of size of the glands and number and length of the salivary ducts, but they are regarded as essentially identical. The detailed differences were not applied to the analysis (see discussion in section V-2). (ii) In Vetigastropoda, the glands are ramified into numerous vermiform tubes and discharge into the buccal cavity through narrow longitudinal openings without duct. (iii) Cocculina, Lepetodrilus, Neomphalus (Pretter et al., 1981), and all neritopsine genera lack salivary glands. The state in Seguenzia is unknown. 62. Esophageal pouches: (0) dorsoventrally depressed lateral pouches [Chi, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Coc, Nem, Ner, Sep, Cin, Wal], (1) absent [Nep, Nau], (2) expanded pouches [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg], (3) posteriorly separated pouches with narrow duct [Pom, Biw] The anterior esophagus is morphologically variable in molluscan groups (Fig. 98). (i) In Polyplacophora, Patellogastropoda, Neritopsina, and Cocculina, the dorsal food channel of the anterior esophagus is accompanied by dorsoventrally depressed, lateral pouches on either side (Salvini-Plawen and Haszprunar, 1987; Salvini-Plawen, 1988). The lateral pouch is elaborated from the central to the posterior region of the buccal mass. The pouches of Neomphalus are also depressed type (Fretter et al., 1981). (ii) Nautilus has a simple tubular canal (Griffin, 1900). Esophagus of Neopilina is also simple, but it develops large paired esophageal diverticula (initially described as a coelom) (Wingstrand, 1985). (iii) In the vetigastropods (including Lepetodrilus and Seguenzia), large pouches expand to cover the posterior part of the buccal mass. (iv) In Ampullariidae (Pomacea) and Pleuroceridae (Biwamelania), the esophageal pouches are separated from anterior esophagus as an oblique sac posterior to the buccal mass.
63. Esophageal gland of mid-esophagus: (0) simple and non-papillate [Chi, Nep, Nau, Coc, Nem, Pom, Biw], (1) septate within mid-esophagus [Pat, Cel, Lim, Pec, Niv, Erg, Nip], (2) papillate [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg], (3) septate and separated posteriorly from esophagus [Ner, Sep, Cin, Wal] Generally, the gastropod mid-esophagus shows a twisted condition due to the torsion, and the interior is lined by glandular epithelium. In the archaeogastropods, the wall of the esophagus expands and develops outgrowths in various ways to increase surface area (Fig. 98). (i) In the outgroups, Cocculina, Neomphalus (Fretter et al., 1981), Ampullariidae (Pomacea), and Pleuroceridae (Biwamelania), the esophageal wall is a simple tube lacking the projecting structure, (ii) In Patellogastropoda, the mid-esophagus is greatly dilated and septated with lamellae, (iii) In all vetigastropod genera, the inner wall of esophagus pouch is papillate. It is also slightly papillate in Seguenzia. (iv) The neritopsine genera have posteriorly separated esophageal glands (=posterior glands of Fretter, 1984 a) that are septated internally. 64. Gastric caecum: (0) absent [Chi, Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Coc, Pom, Biw], (1) large and bulbous [Nau], (2) large and spiral [Mik, Sul, Tur, Chl, Sto], (3) small and crescent-shaped [Scu, Mac, Ana, Bro, Lep, Seg, Nem, Ner, Sep, Cin, Wal ] The stomach of many molluscs is provided with a caecum of various grade of development. Its variation is divided into the four discrete states. (i) The gastric caecum is absent in Polyplacophora (Fretter, 1937; Salvini-Plawen, 1988) and Tryblidiida (Lemche and Wingstrand, 1959), Patellogastropoda Cocculina, Pomacea (Andrews, 1965; Berthold, 1991) and Biwamelania. (ii) In Nautilus, the caecum is a simple sac but well developed (Griffin, 1990: fig. 23). (iii) Multispiral type is prominent in the stomach of Pleurotomariidae, Haliotidae, and most trochoids (Turbo, Chlorostoma, and Stomatia). (iv) Short, crescent type is found in Fissurellidae, Anatoma, Seguenzia, Lepetodrilus, Broderipia, Neomphalus (Fretter et al., 1981), and in all neritopsine groups. 65. Style sac region, including sorting area and tooth of gastric shield: (0) absent [Chi, Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip], (1) present [Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] The style sac region of the stomach generally consists of a cuticularized gastric shield and its tooth, plus a ciliated sorting area between the typhlosoles. In Polyplacophora, the ciliated area is present in the stomach, but is not as well organized as in the distinct area (Fretter, 1937; Salvini-Plawen, 1985, 1988). It is totally absent in the modified stomach of Tryblidiida (Lemche and Wingstrand, 1959). In the stomach of Nautilus, there are two longitudinal ridges with transverse furrows between them (Griffin, 1900: fig. 32). These ridges are continuous with the entrance of the caecum, suggesting that they are homologous with the typhlosoles of other mollusks (the topological right is the minor typhlosole, the left is the major typhlosole). Likewise, the median corrugated area is homologous with the sorting area of other groups. In gastropods, this region is well-developed and shows rather similar morphology at least within archaeogastropods except Patellogastropoda (Fig. 99). The stomach of all patellogastropod genera lacks a style-sac region almost completely. The absence of all inner structure except typhlosoles suggests the only the proximal part of the stomach is retained near the openings of the digestive glands, and that the initial part of the intestine is expanded to form most of the "stomach" (Fretter, 1990).
66. Anterior loop of intestine: (0) absent [Chl, Nep, Nau, Biw], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom] The so-called anterior loop, a particular course of gut convolution, is shared by archaeogastropod genera. After departure from the stomach, the intestine extends anteriorly to the right posterior region of the buccal mass, crossing over the esophagus. This general pattern is maintained in archaeogastropods, including Neomphalus (Fretter et al., 1981), but does not occur in "higher" Caenogastropoda. Ampullariidae of Architaenioglossa also possess a short loop over the esophagus, which is comparable to the archaeogastropod condition. In the outgroups, the intestine is coiled only posteriorly in Polyplacophora (Fretter, 1937) and Neopllina (Lemche and Wingstrand, 1959), or is simply bent apart from the anterior region in Nautilus (Griffin, 1900). 67. Course of the rectum relative to the pericardium: (0) rectum penetrating neither ventricle nor pericardium [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Seg, Coc, Nem, Wal, Pom, Biw], (1) rectum penetrating both ventricle and pericardium [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bra, Lep, Ner, Sep, Cin] The relationship between the pericardium and the course of the rectum (=posterior region of the intestine) is variable in molluscan groups. The rectum runs freely on the ventral side of pericardium in Polypla cophora (Hyman, 1967; Salvini-Plawen, 1985; Eernisse and Reynolds, 1994) and in Nautilus (Griffin, 1900). In Neopilina, the rectum passes between two isolated pericardial sacs, each of which includes a ventricle and two atria (Lemche and Wingstrand, 1959: fig. 143). Among gastropods, the rectum does not pass through the pericardium in Patellogastropoda, Cocculina, Waldemaria, Neomphalus (Fretter et al., 1981) and Caenogastropoda, whereas penetration occurs in all zeugobranchs, Trochoidea, Lepetodrilus, and neritopsine genera excluding Waldemaria. It is a general pattern that the rectum always passes through the ventricle when it penetrates the pericardium in the latter groups. 4. Circulatory System68. Number of auricles: (0) single pair [Chi, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep], (1) two pairs [Nep, Nau], (2) single pair with right auricle nonfunctional [Ner, Sep, Cin], (3) single left auricle (unpaired) [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Seg, Coc, Nem, Wal, Pom, Biw] Molluscs generally have a median ventricle and paired auricles, but the number of auricles is variable among different taxa. Among the outgroups, Polyplacophora have a pair of auricles (Hyman, 1967; Salvini-Plawen, 1985; Eemisse and Reynolds, 1994). Nautilus has two pairs of auricles that are associated with two paired ctenidia and efferent ctenidial vessels (Griffin, 1900). Neopilina also has two pairs of auricles (Lemche and Wingstrand, 1959). Among Gastropoda, Patellogastropoda, Cocculina, Seguenzia (Quinn, 1983; Ponder and Lindberg, 1997), Neomphalus (Pretter et al., 1981), Waldemaria, ampullariids, and Caenogastropoda have a single auricle (monotocardian condition). Only the vetigastropod genera and Lepetodrilus have a truly diotocardian heart with two functional auricles, but neritopsine genera excluding monotocardian Waldemaria have a single pair with right auricle nonfunctional. 69. Bulbous aorta: (0) undifferentiated or simple extension of aorta [Chi, Nep, Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) developed into muscular sac [Pat, Cel, Lim, Pec, Niv, Erg, Nip] The muscular aortic bulb is elaborated within the pericardium of Patellogastropoda. It rhythmically contracts to send blood to both anterior and posterior aortae as an accessory pump of the heart (Jones, 1968). The development of such a contractile apparatus is not found in the aorta of any other group. The ampulla of Ampullariidae is a clearly independent structure because it originates from part of the anterior aorta and has no relationship to the posterior aorta. 70. Basibranchial sinus: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) developed [Mik, Sul, Scu, Mac], (?) unknown [Ana] The blood space occurring in the anterior portion of the efferent renal vessel is specially expanded as a 'basibranchial sinus' in zeugobranch genera (Fretter and Graham, 1962: fig. 147). The state in Anatoma (with paired ctenidia) could not be verified. 71. Transverse pallial vein: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Tur, Chl, Sto, Bro, Lep], (?) unknown [Seg] In some vetigastropods, the vessel from the right kidney to the afferent ctenidial vessel crosses the mantle skirt and the left hypobranchial gland transversely (e.g. Fig. 46 a). This particular section of the vessel is termed the transverse pallial vein (Fretter and Graham, 1962). It is present in Trochidae, Turbinidae, and Lepetodrilus, but is absent in all other groups. The state in Seguenzia could not be verified.
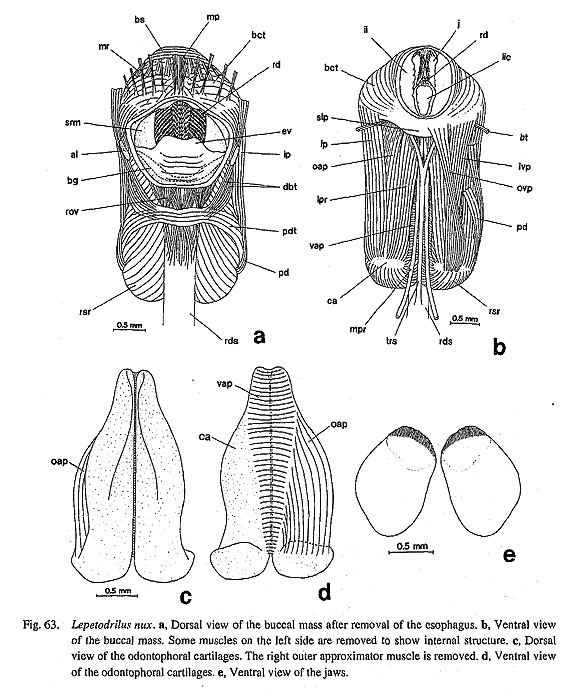
5. Excretory System72. Number of kidneys: (0) single symmetrical pair [Chi], (1) six pairs [Nep], (2) two pairs [Nau], (3) single asymmetrical pair [Pat, Cel, Lim, Pec, Niv, Erg. Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg], (4) left only [Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] The number of kidneys (nephridia) and kidney openings (nephridiopores) is extremely variable in the outgroups: a single pair in Polyplacophora (Eemisse and Reynolds, 1994) and two pairs in Nautilus (Schipp and Martin, 1987). In Tryblidiida, excretory organs comprise six pairs in Neopilina, seven pairs in Vema, and three to four pairs in Micropilina (Lemche and Wingstrand, 1959; Wingstrand, 1985; Haszprunar, 1997). In general, the paired right and left sides of molluscan excretory organs are bilateral and not differentiated in form and function. Exceptionally, gastropods have an asymmetrically paired or unpaired kidney. Most Archaeogastropoda have paired kidneys, whereas Cocculina (and generally Cocculinoidea; Haszprunar, 1988 c), Neomphalus, Neritopsina, and Caenogastropoda have single left kidney. The state in Seguenzia was scored as "paired," following Ponder and Lindberg's (1997) data matrix (character 29). 73. Position of the kidneys relative to the pericardium: (0) left and right kidneys on either side of pericardium [Chi, Nep, Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg], (1) both left and right kidneys on the right side of the pericardium [Pat, Cel, Lim, Pec, Niv, Erg, Nip], (2) post-torsional left kidney on the right side of the pericardium [Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] Ponder and Lindberg (1997) pointed out that the position of kidney and pericardium show discrete states throughout molluscan groups. In Patellogastropoda, both kidneys characteristically lie on the right side of the pericardium. In Vetigastropoda, including Lepetodrilus, the left kidney is suspended from the mantle skirt on the anterior left side of the pericardium, while the right kidney is extended to visceral region on posterior right side of the pericardium. Similarly, the outgroups have paired kidneys located symmetrically on either side of the pericardium. The remaining groups have a single left kidney on the right side of the pericardium. 74. Papillary sac: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) developed [Mik, Sul, Tur, Chl, Sto, Bro], (2) small with indistinct papillae [Scu, Mac], (?) unknown [Ana] In most vetigastropod groups, the inner surface of the left kidney is evaginated to form papillate surface (the so-called papillary sac). Functionally, this specialization generates a high surface-volume ratio of epithelia to lumen of the kidney. This state is present in Pleurotomariidae, Haliotidae, Turbinidae, and Trochidae, but is not differentiated in the remaining ingroup taxa and the outgroups. In Fissurellidae, the left kidney is greatly reduced in size, but histologically regarded as homologous with the typical papillary sac (Andrews, 1985; 1988). The state in Seguenzia was scored as "absent" based on Ponder and Lindberg's (1997) data matrix (character 26). Histological aspects were have not been adequately investigated in Anatoma. 6. Reproductive System6-1. General Characters75. Position of gonad: (0) dorsal to digestive glands [Chi, Nau, Mik, Sul, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) ventral to digestive glands [Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Scu, Mac, Coc] The position of the gonad within the visceral mass has been regarded as a phylogenetic character for higher-level molluscan phylogenetics (Ponder and Lindberg, 1996; 1997). The gonad is most of ten located dorsal to the digestive glands and therefore occupies the highest position within the visceral mass. Polyplacophora, Nautilus, and most Gastropoda all retain such a topological relationship. However, the reversed position occurs in Tryblidiida (Lemche and Wingstrand, 1959), Patellogastropoda, Fissurellidae, and Cocculina (Fig. 71). The gonad is ventrally spread and concealed by the overlapping digestive glands in these forms. In Neomphalus, the gonad is situated dorsally (Fretter et al., 1981:figs. 12, 13).
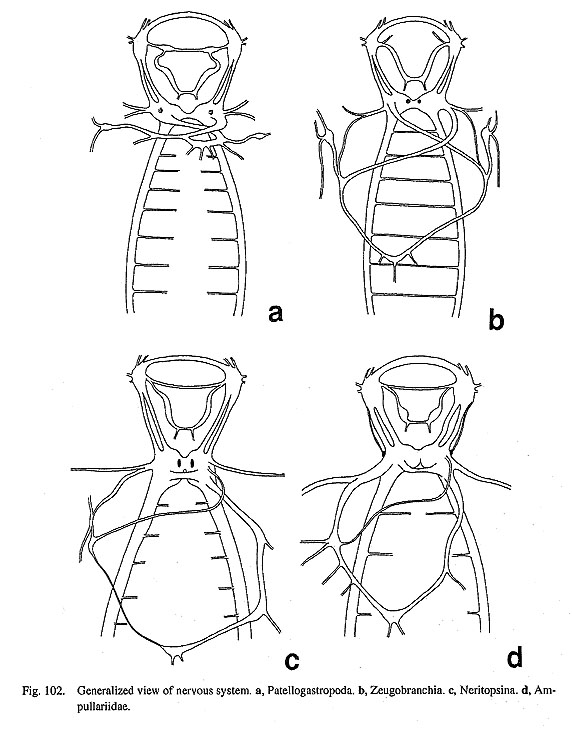
76. Number of gonads and gonoducts: (0) paired [Chi, Nep], (1) pre-torsional right only [Nau], (2) post-torsional right (= pre-torsional left) only [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] The numbers of the gonads and gonoducts are variable in molluscs at higher taxonomic levels (Ponder and Lindberg, 1996; 1997). Polyplacophora have a single median gonad with bilaterally separated gonoducts (Hyman, 1967). Neopilina have two pairs of gonads, each of which is connected with the excretory organ through its own gonoduct (Lemche and Wingstrand, 1959). In Nautilus, the gonad itself is symmetrical, but the gonoduct exists only on the pre-torsional right side in both sexes. However, the gonoduct may be originally paired because there is vestigial structure that is indicative of originally paired condition (Arnold, 1987). In all Gastropoda, both the gonad and gonoduct are asymmetrical, being an the posttosional right side only. 77. Opening site of the gonoducts: (0) directly into pallial cavity [Chi, Nau, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) through kidney into pallial cavity [Nep, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep] Polyplacophora and Nautilus have gonoducts which are independent of the kidney, while those of Neopilinidae have a direct connection with distal section of the excretory organ (Lemche and Wingstrand, 1959). Among Gastropoda, most archaeogastropods including Lepetodrilus discharge gametes through the right kidney (Fig. 100). However, hermaphroditic Cocculina has a separated glandular gonoduct (Figs. 69 a, 72 d), and all neritopsines and caenogastropods have an independent, well-developed pallial gonoduct. The gonoduct of Seguenzia was scored as independent of kidney, following Ponder and Lindberg's (1997) data matrix (character 33). Neomphalus also has an isolated gonoduct in both sexes (with a gonopericardial duct only in female) (Pretter et al., 1981).
6-2. Female Organ78. Pallial oviduct composed of paired albumen glands and capsule gland: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Wal, Pom, Biw], (1) present [Ner, Sep, Cin] Most archaeogastropods (excluding neritopsine genera) release gametes through the right kidney, and accordingly females lack a well-developed pallial gonoduct. In contrast, the female gonoducts in Neritopsina and Caenogastropoda are elaborated into a glandular oviduct, consisting of a proximal albumen gland and a distal capsule gland. However, it is very difficult to establish the homology of individual structure constituting pallial oviduct throughout gastropod higher taxa, as pointed out by Ponder and Lindberg (1997). Exceptionally only some neritopsine genera within Archaeogastropoda seem to share a characteristic and homologized set of paired albumen glands and capsule gland (Fig, 101). In other archaeogastropods, the true (not urogenital) oviduct of Neomphalus, Cocculina, and Seguenziidae do not develop glands comparable to those of neritopsines.
79. Number of female genital openings: (0) monaulic [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik. Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Pom, Biw], (1) diaulic [Ner, Cin, Wal], (2) triaulic with enigmatic duct [Sep] A vaginal opening independent of the female gonopore is a common and unique feature in neritopsine genera within Archaeogastropoda (Fig. 101). Especially in the genus Septalia, an additional accessory duct of unknown function (so-called "ductus enigmaticus") is separated from the vaginal duct and extended back to the pallial cavity (Fig. 101 b, ed). Therefore, the gonad is monaulic with a single reproductive opening in all non-neritopsine groups, but diaulic in most Neritopsina and uniquely triaulic in Septalia. Arnpullariidae have a semi-closed monaulic pallial oviduct (Andrews, 1964; Berthold, 1991), whereas Cerithioidea including Pleuroceridae have an open pallial oviduct with a longitudinal cleft (Houbrick, 1988). 80. Spermatophore sac: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Wal, Pom, Biw], (1) present [Ner, Sep, Cin] In neritopsine genera except for Waldemaria (Helicinidae), a special bursa for the reception of a Spermatophore is developed within the gonoducts. The sac is distended by the Spermatophore after copulation during the breeding season. In Nerita, the thin tip of the Spermatophore extends from this sac into the duct to seminal receptacle for sperm transfer (Fig. 76 d). This structure is functionally identical to the "bursa copulatrix" of other groups, but homologies are doubtful among gastropod higher taxa (Ponder and Lindberg, 1997).
81. Crystal sac: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Cin, Wal, Pom, Biw], (1) present [Ner. Sep] The neritid genera (Nerita and Septalia) deposit lenticular egg capsules with reinforcement minerals (Andrews, 1935; Fretter, 1946; Fretter and Graham, 1978; Bandel, 1982; Houston, 1990). The minerals are secreted by and stored in the crystal sac of the gonoduct. When minerals are being stored, this sac is easily recognized through the thin mantle by the color of minerals. Other groups, including Phenacolepadidae and Helcinidae, do not possess such a mineral-secreting organ. 6-3. Male Organ82. Cephalic penis on the inner side of the right cephalic tentacle: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Wal, Pom, Biw], (1) present [Ner, Sep, Cin] Most neritopsine genera (except Waldemaria) have a cephalic penis on the inner side of the right cephalic tentacle. Although various archaeogastropods, especially deep-sea taxa, develop a copulatory apparatus in the head region, they are not homologous with the penes of neritopsines due to a difference in position. The so-called "penis" of Erginus (Golikov and Kussakin, 1972; ="subcephalic tentacle" in this study; Fig. 20) is the only structure comparable topologically to neritopsine penes. However, they are not homologous because of great differences in structure (ciliated tentacle without duct or groove in Erginus vs. smooth penis with lateral groove in Neritoidea). 83. Seminal vesicle derived from vas deferens; (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Wal, Pom, Biw], (1) present [Ner, Sep, Cin] In the neritopsine genera except Waldemaria, the vas deferens is heavily coiled to form a mass of folded ducts which acts as a seminal vesicle. The formation of a Spermatophore seems to be initiated in this structure, The seminal vesicle of Lepetodrilus (Fretter, 1988) is not regarded as homologous because it has much less coiling form than those of Neritoidea. Males of Neomphalus (Fretter et al., 1981) and other taxa do not exhibit such a specialization. 84. "Annex gland" on the prostate: (0) absent [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Wal, Pom, Biw], (1) present [Ner, Sep, Cin] The prostate is widely covered by a gland called the "annex gland" in neritoid genera (Berry et al., 1973). No other groups are found with a comparable glandular area, but the functional significance of the gland is still unknown. Another gland in the male neritid pallial gonoduct, the "basal gland" (Berry et al., 1973), is found only in the genus Nerita even within Neritidae, and therefore it is not concerned with the analysis at the suprafamilial level. 7. Nervous System7-1 Nerve Ring85. Position of pleural ganglia: (0) pleural ganglia not clearly separated [Chi, Nep, Nau], (1) hypoathroid (pleural ganglia in ventral position) [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sen, Cin, Wal, Pom], (2) epiathroid (pleural ganglia in dorsal position) [Biw] The circumesophageal nerve ring of the outgroups is markedly differentiated as compared with those of gastropods, although they consist of comparable parts. Polyplacophora and Tryblidiida have the amphineurous-type nervous system composed of a circumesophageal nerve ring and lateral and ventral nerve cords. The central nervous system of Nautilus is less concentrated than typical coleoid brain, but it is made of a tight nerve ring and short palliovisceral cord (Griffin, 1900). In any case, the pleural ganglia of the outgroups are not distinctly separated from other ganglia. All genera of Archaeogastropoda investigated have the so-called hypoathroid condition in which pleural ganglia are distant from cerebral ganglia and juxtaposed to pedal ganglia (Fretter and Graham, 1962). In the architaenioglossate groups, an intermediate "dystenoid" condition is known in Vivipariidae (Fretter and Graham, 1962), but the Ampullariidae have typical hypoathroid condition (Fig. 102). In Neotaenioglossa of Caenogastropoda, the central nervous system is epiathroid in which the pleurals are closely associated with the cerebrals rather than the pedals.
7-2. Buccal Region86. Labial ganglia: (0) absent [Chi, Nep, Nau, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip] Labial ganglia are present in all patellogastropod limpets hitherto investigated (e.g. Figs. 4 c, 9). They are positioned beneath the entrance to the sublingual pouch and give off nerves anteriorly toward the ventral labial region and posteriorly to the labiobuccal connectives. Homologous ganglia have not been found in other molluscan groups.
87. Labial/subcerebral commissure: (0) present [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Ner, Sep, Cin, Wal, Pom], (1) absent [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Coc, Nem, Biw], (?) unknown [Seg] A labial commissure is clearly present in all species of Patellogastropoda, Neritopsina and Ampullariidae, but it is absent in other groups of Archaeogastropoda and Caenogastropoda. Neomphalus also lacks this commissure (Fretter et al., 1981). The states in Seguenzia is unknown. Compared with nervous system of outgroups, labial commissure of gastropods can be regarded as homologous with the "subcerebral commissure" (Eernisse and Reynolds, 1994) of the amphineurans. They originate from the cerebral ganglia and are connected ventral to the alimentary canal. 7-3. Lateral/Visceral Loop88. Position of lateral/visceral nerve loop: (0) outside shell muscles [Chi, Nep], (1) inside shell muscles [Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac. Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] In the amphineuran nervous system of Polyplacophora and Tryblidiida, the lateral nerve cord runs outside of the shell muscles and is connected transversely with the ventral cords by commissures. In Nautilus and gastropod genera, the corresponding visceral loop lies inside the shell muscles (Haszprunar, 1988 b; Ponder and Lindberg, 1997). 89. Streptoneury of lateral/visceral nerve loop: (0) absent [Chi, Nep, Nau], (1) present [Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw] The twisted visceral nerve loop is one of the manifest results of gastropod torsion. This condition is restricted to gastropods and is shared at least by all archaeogastropods and other "prosobranchs." The contrasting state is, however, found in Heterobranchia as untwisted configuration of visceral loop which is caused by "detorsion" and/or several other secondary modifications (Brace, 1977; Schmekel, 1985; Haszprunar, 1988 b: Mikkelsen, 1996). In archaeogastropods, Streptoneury occurs regardless of shell form and size, as is shown in purely symmetrical patellogastropod limpets. 90. Origin of lateral/visceral loop: (0) from left and right pleural ganglia [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Scu, Mac. Ana, Tur. Chl, Sto, Bro, Lep, Seg, Coc, Nem, Biw], (1) only from right ganglion [Ner, Sep, Cin, Wal, Pom] The lateral/visceral nerve loop of various molluscan groups normally arises from both right and left pleural ganglia. However, all neritopsine genera and ampullariids (Berthold, 1991) have a quite different pattern of the visceral nerve loop (Figs. 102 c, d). The loop originates only from the right pleural ganglion, and is seemingly untwisted for the most part. This unusual condition was interpreted to have been achieved by the anterior shift and fusion of the subesophageal ganglion with the pleural ganglion (Berthold, 1991: figs. 344-347). In Mikadotrochus, the loop is connected to cerebropleural connectives rather than to pleural ganglia directly. This state in Mikadotrochus is probably caused by a partial fusion of cerebropleural connectives and visceral loop and represents a slightly modified condition of normal two-sided origin. 7-4. Pedal Region91. Median commissure of the pedal cords: (0) absent [Chl, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) present [Scu, Mac] Scutus and Macroschisma of Fissurellidae share a special condition in the pedal cords. The left and right cords are connected by a thick transverse connection in the central part of the foot in addition to typical pattern of thin commissures (Figs. 36 f, 41 c). No similar configuration of the pedal nervous system is found in other molluscan groups.
92. Position of the pedal (ventral) cords: (0) embedded in pedal musculature [Chi, Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Mik, Sul, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal, Pom, Biw], (1) exposed on pedal musculature [Scu, Mac] Other unusual aspects of the nervous system are seen in Fissurellidae. The entire pedal cords are naked on the pedal musculature beneath visceral mass, and so that parallel thin pedal nerves are also exposed on the musculature in the visceral cavity. Such conditions never occur in other molluscan groups. "Normally" the pedal cords are buried completely in the foot. 93. Position of statocysts: (0) absent [Chi], (1) lateral to pedal ganglia on outer sides [Nep, Nau, Pat, Cel, Lim, Pec, Niv, Erg, Nip, Pom, Biw], (2) anterodorsal or dorsal to pedal ganglia [Mik, Sul, Scu, Mac, Ana, Tur, Chl, Sto, Bro, Lep, Seg, Coc, Nem, Ner, Sep, Cin, Wal] The possession of statocysts is a shared character of the conchiferan classes. They are commonly innervated from the cerebral ganglia regardless of their position (Wingstrand, 1985). Among the outgroups, Neopillna has statocysts on the outer sides of the nerve ring (Lemche and Wingstrand, 1959). In Nautilus, they are situated on the outer posterolateral sides of pedal ganglia (Young, 1987: figs. 2,4). In archaeo- and caenogastropods, two different positions are observable (Fig. 102). (i) Statocysts occur on the outer sides of the pedal ganglia in patellogastropod limpets, Ampullariidae (Pomacea), and Pleuroceridae (Biwamelania). (ii) Statocysts are attached to the dorsal or anterodorsal surfaces of the pedal ganglia in Vetigastropoda, Cocculina, Neomphalus (Fretter et al., 1981), and Neritopsina. IV-2. Cladistic AnalysisMost parsimonious trees: The cladistic analysis, using a heuristic search on the data matrix (Table 3; unordered and unweighted data) yielded four equally parsimonious trees of length 209 steps (CI= 0.660, RI = 0.881, RCI= 0.582) (Fig. 103). All trees obtained were of similar topology with only slight differences. In the resulting strict consensus tree (Figs. 104), polytomies were caused by three unresolved relationships, namely relationships among Acmaeoidea, Zeugobranchia, and Trochoidea. All other parts of the tree were fully resolved.
The relationships of the clades are summarized as follows (Fig. 104): (1) Monophyly of Gastropoda (node 3) is supported. (2) The Gastropoda is divided into Patellogastropoda (node 4) and nonpatellogastropod groups, namely, Orthogastropoda (node 9). (3) Within Patellogastropoda, Lepetidae (Limalepeta) branches off first. Then the remaining groups (node 5) form two small clades, Patelloidea (node 6), and Acmaeoidea (node 7). (4) The orthogastropod clade bifurcates into two clades, Rhipidoglossa (node 10) and Caenogastropoda (node 24). (5) The Rhipidoglossa is composed of Neritopsina (node 21) and non-neritopsine (node 11) clades. The latter group does not have a tax on name, but corresponds to Archaeogastropoda (s.s.) of Ponder and Lindberg (1997). (6) Within Neritopsina, Helcinidae (Waldemaria) is separated as an independent clade. The remaining three genera (Cinnalepeta, Nerita, and Septalia) form another clade, Neritoidea (node 22). Nerita and Septaria are united as a clade corresponding to Neritidae (node 23). (7) The remaining rhipidoglossate groups, excluding Neomphalus and Cocculina, are united as Vetigastropoda (node 13) which consists of Seguenzia, Lepetodrilus, and a clade (node 15) of Zeugobranchia (node 16) plus Trochoidea (node 19). (8) Within Zeugobranchia, clades are always formed by Fissurellidae (Scutus and Macroschisma) (node 18) and pleurotomarid-haiotid genera (Mikadotrochus and Haliotis) (node 17). The position of Anatoma was not resolved. Scaled analysis: The characters coded by more than two character states theoretically have greater influence on tree topology than binary characters, in proportion to the number of assigned states (Swofford and Begle, 1993). In this analysis, 66 characters are binary, but the data set includes 16 characters having 3 states and 11 characters having more than 3 states. Therefore, multistate characters in this analysis are expected to have significant effects. An analysis was performed based on the characters given 1000 base weight to estimate their effect on tree topology. They were all changed into equally weighted state by scale option. The scaled analysis resulted in two most-parsimonious trees. Their strict consensus tree (Fig. 105 a) is congruent with that of the unsealed analysis (Fig. 104), and all the major clades retained the same topology as that of unsealed analysis. This indicates that influence of multistate characters was insignificant for tree topology.
Bootstrap analysis: Bootstrapping probabilities were calculated to show which nodes of the cladogram are significantly supported by the data presented. It should be noted that this method includes some assumptions to accept the result as an effective estimate of confidence limits (Pelsenstein, 1985; Sanderson, 1989, 1995): (i) Character state changes must be independent. (ii) The observed data set must represent a universal pattern of distribution. However, changes in morphological character states are not always independent, and morphological data always contain bias due to subjective observation and limited taxonomic coverage. Thus, the probabilities derived from this analysis should not be overestimated as an indicator of confidence. 100 bootstrap replicates were attempted by heuristic analysis with random addition sequence of 30 replicates and branch-swapping by tree bisection reconnection (TBR). In the 50% majority-rule consensus tree of the bootstrapping (Fig. 105 b), monophyly of Patellogastropoda, Orthogastropoda, Neritopsina, Vetigastropoda, "Zeugobranchia+Trochoidea," Trochoidea, and Caenogastropoda were all confirmed by high val ues between 93 and 100. Clades representing Rhipidoglossa, "Cocculina+Vetigastropoda" and "Lepetodrilus +Zeugobranchia+Trochoidea" were relatively well supported by values between 79 and 70. In contrast, subclades within Patellogastropoda, non-neritopsine Rhipidoglossa, and Zeugobranchia had relatively low bootstrap values. Decay analysis: Cladograms of one or two steps longer than the shortest were calculated to assess the relative robustness of the topology. The analysis yielded 187 trees of length 210 and 2418 trees of length 211. (1) Trees with one additional step (length = 210) (Fig. 105 c): Patellogastropoda, Rhipidoglossa, Neritopsina, "Coccylina+Vetigastropoda," Vetigastropoda, "Zeugobranchia+Trochoidea," and Caenogastropoda were supported as monophyletic groups. In contrast, the relationships within patellogastropods and vetigastropods and the basal part of Rhipidoglossa collapsed into polytomies, except for three terminal clades of Patelloidea (Patella and Cellana) and Fissurellidae (Macroschisma and Scutus), and Trochoidea (Turbo, Chlorostoma, Stomatia, and Broderipia). p>(2) Trees with additional two steps (length = 211) (Fig. 105 d): Compared with the former tree, some parts of tree topology showed major changes. Further clade decays occurred (i) in basal part of Orthogastropoda (Caenogastropoda+Neritopsina+Neomphalus+Cocculina+Vetigastropoda) and (ii) in "Zeugobranchia+Trochoidea." These were collapsed into a complete polytomy.Robustness of tree topology: The results using different analysis options did not reveal surprising differences or significant contradictions. The suggested relationships are summarized as follows: (1) Strong support was obtained for monophyly of Patellogastropoda and for a basal division of gastropods into Patellogastropoda and Orthogastropoda. (2) The bifurcation of Orthogastropoda into Caenogastropoda and Rhipidoglossa clades is possible with relatively high reliability. (3) Neritopsina are united as a very robust clade. (4) The monophyly of non-neritopsine Rhipidoglossa is not very reliable. Position and relationships of Neomphalus and Cocculina also cannot be taken as conclusive. (5) Vetigastropoda and "Zeugobranchia+Trochoidea" fonn a robust clade. (6) The two caenogastropod genera were monophyletic and independent of archaeogastropod genera. |