CHAPTER VI
Physicochemical Environment of Au-Ag Mineralization
|
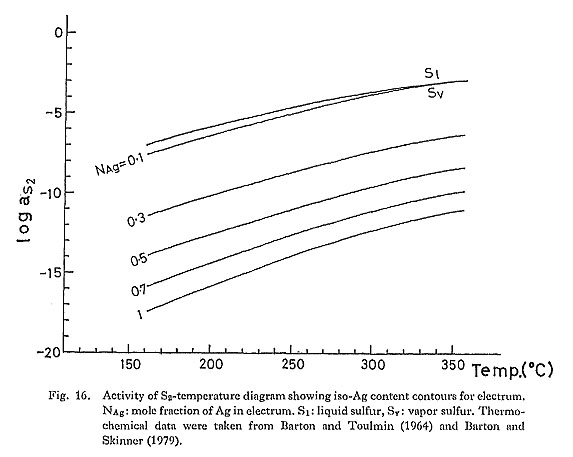
As discussed in Chapter V, the chemical composition of electrum is controlled by several physicochemical variables. Therefore, the chemical composition of clectrum coexisting with opaque and gangue minerals, and fluid inclusion data can be used to deduce the physicochemical environment of Au-Ag mineralization. 1. Activity of Sulfur and TemperatureWhen argentitc (or acanthite) is in equilibrium with electrum, the following reaction can be used to derive the relationship among aS2, temperature, and Ag content of electrum. 2(Ag) + 1/2S2 = Ag2S (22) Using equilibrium relation for the reaction (22), iso-mole fraction contours for the Ag component in electrum are drawn on a log aS2-temperature diagram (Fig. 16). NAg increases with decreasing aS2, at a given temperature. The FeS content of aphalerite in equilibrium with iron sulfides (pyrite, pyrrhotite) is also useful to place a limit on aS2 and temperature ranges (Barton and Toulmin, 1966), as shown in Fig. 17.
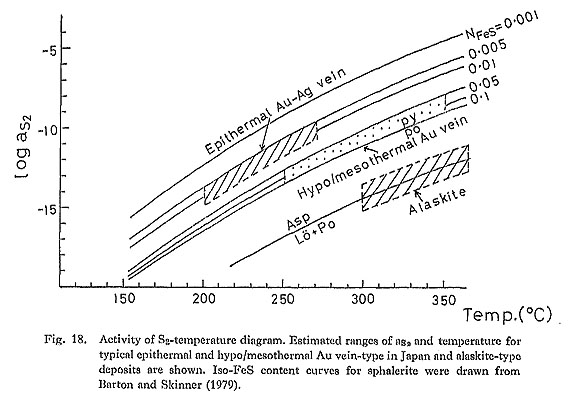
When the Ag content of electrum coexisting with argentite is combined with the FeS content of sphalerite, the ranges of aS2 and temperature can be restricted. This electrum-sphalerite geothermometry has been applied to epithermal Au-Ag vein-type deposits in Japan (e.g., Shikazono, 1985b). Shikazono (1985b) has shown that this geothermometry is highly useful for the determination of aS2 and temperature in. the temperature range of about 180°C-300°C. Estimated ranges of aS2 and temperatures of hypo/mesothermal vein-type deposits in Japan and in, the Korean Peninsula and alaskite-type deposits arc shown in Fig, 14. These ranges were estimated by using the FeS content of aphalerite, the Ag content of electrum, several sulfidation reactions and homogenization. temperatures of fluid inclusions. It can be seen in Fig. 18 that aS2 for epithermal vein-type deposits is higher than that for hypo/mesothermal vein-type deposits and alaskite-type deposits. Homogenization temperatures of fluid inclusions for hypo/mesothermal vein-type and alaskite-type deposits are not equal to the temperature of formation. The pressure effect must be taken into account to correct the homogenization temperature to obtain the true formation temperature. If total pressure for hypo/mesothermal vein-type deposits is about 0.5 Kb that was estimated from geologic evidences (Nedachi, 1974; Shelton, 1986), about 30°C must be added to homogenization temperature in order to obtain the temperature of formation. Total pressure at the site where alaskite-type deposits formed has not been estimated. Temperature that must be corrected for homogenization temperature of epithermal vein-type deposits is mostly less than 20° (Shikazono, 1985a). Many fluid inclusion data on epithermal Au-Ag vein-type deposits are available (e.g., Enjoji and Takenouchi, 1976; Shikazono, 1985a). In contrast, few data on fluid inclusions for hypo/mesothermal vein-type and alaskite-type deposits are obtained. However, the fluid inclusion studies suggest that an increasing order of temperature of formation is as follows: epithermal Au-Ag vein-type, hypo/mesothermal vein-type, and alaskite-type deposits.
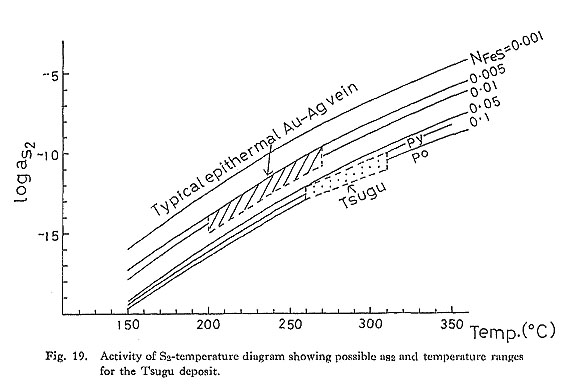
The possible ranges of aS2 and temperature for the Au deposits associated with Hg and Sb mineralization (Tsugu and Sakoshi-Odomari) are also shown in Figs. 19 and 20. It is interesting to note that the range of aS2 for these deposits is lower than that for typical epithermal Au-Ag vein-type deposits at a given temperature.
Shikazono (1986) classified the epithermal deposits in Japan into three subtypes; epithermal Au disseminated, epithermal Au-Ag vein-type, and epithermal base metal vein-type deposits. Remarkable contrasts in mineralogy are present among these types of deposits. For instance, the FeS content of sphalerite from epithermal Au-Ag vein-type deposits is below 1 mole %, whereas that from epithermal base metal vein-type deposits is widely variable and is generally more than 1 mole % (Shikazono, 1977b). There is a difference in the occurrence of iron minerals in these deposits. Pyrrhotite is sometimes found in epithermal base metal vein-type deposits (e.g., the Toyoha, Oe, and Oizumi deposits), but it is absent in epithermal Au-Ag vein-type deposits. Through the use of FeS content of sphalerite, Ag content of electrum, homogenization temperatures of fluid inclusions, and mineral assemblages, typical aS2-temperature ranges for these three types of deposits have been estimated (Shikazono, 1986; Fig. 21). It can be seen in Fig. 21 that aS2 for epithermal Au-Ag vein-type deposits is higher than for epithermal base metal vein-type deposits.
As already described, a small amount of electrum occurs in epithermal base metal vein-type deposits. Electrum cannot be observed in the epithermal base metal vein-type deposits in which pyrrhotite occurs (e.g., Toyoha-Soya, Oizumi, and Hosokura). However, electrum is found in epithermal base metal vein-type deposits in which hematite is commonly observed (e.g., Osarizawa and Ani). Motomura (1986) noted that the Ag content of electrum from epithermal base metal vein-type deposits (e.g., Oe, Inakuraishi, Jokoku, and Imai Ishizaki) is slightly higher than from epithermal Au-Ag vein-type deposits. The above mineralogical features suggest that aS2 for epithermal base metal vein-type deposits associated with Au mineralization is higher than for epithermal base metal vein-type deposits that are not associated with Au mineralization, as shown in Fig. 21.
2. ∑Au/∑AgUsing the equation (3) and giving values of aH2S, pH, and NAg/NAu, Shikazono and Shimizu (1987) calculated ∑Au/∑Ag in epithermal ore-forming fluids in which Au(HS)2- and AgCl2- are predominant Au and Ag species to be 10-1 to 10-2. If AgCl2- and AuCl2- are dominant in hypo/mesothermal ore-forming fluids, ∑Au/∑Ag can be calculated from the equation (6). ∑Au/∑Ag calculated by Shikazono and Shimizu (1987) for hypo/mesothermal ore-forming fluids is around 10-1. |