CHAPTER V
Factors Controlling Chemical Compositions of Electrum
1. Ag Content1) Factors Controlling Ag ContentThe Ag content of electrum is dependent on the chemical states of Au and Ag in oreforming fluids. Great efforts to clarify these states have been made mainly through the solubility experiments of Au and Ag minerals in hydrothermal solutions (e.g., Barnes and Czamanske, 1967; Seward, 1973, 1976a, b, 1983; Barnes, 1978; Shenberger, 1985). According to these studies, several chemical states of Au and Ag in ore-forming fluids are considered to be stable. Seward (1973) has experimentally revealed that dominant chemical states of Au in epithermal ore-forming fluids and active geothermal waters (e.g., Broadlands in New Zealand) are Au thio-complexes among which Au(HS)2- is usually dominant in epithermal ore-forming fluids. It is also possible that AuCl2- concentration is higher than Au(HS)2- concentration in orc-forming fluids responsible for some epithermal vein-type deposits (Casadevall, 1976). However, Au(HS)2- is considered to be more abundant than AuCl2- in ore-forming fluids responsible for epithermal Au-Ag vein-type deposits in Japan (Shikazono, 1974c; Hattori, 1975; Ichikuni, 1981; Shikazono and Shimizu, 1987). Dominant Ag species in epithermal ore-forming fluids may be Ag chloride complexes (e.g., AgCl2-; Seward, 1976b). But it is also possible that Ag bisulfide complex (Ag(HS)2-) is stable in epithermal ore-forming fluids, considering the high solubility of Ag2S due to bisulfide complexes in hydrothermal solution (e.g., Melent'yev et al., 1969). Unfortunately, thermochemical data on Ag bisulfide complexes at elevated temperatures of concern have not yet been published. In ore-forming fluids responsible for Japanese hypo/mesothermal Au vein-type deposits, AuCl2- may be dominant among Au species (Shikazono and Shimizu, 1986, 1987), although Au(HS)2- might also be stable in low temperature regions (probably below ca. 250°C to 300°C). AgCl2- may be dominant in the hypo/mesothermal ore-forming fluids (Shikazono and Shimizu, 1987). There are several other possible stable chemical states of Au and Ag in epithermal and hypo/mesothermal ore-forming fluids: arsenic, tellurium, selenium, bismuth and antimony complexes. However, thermochemical data on these aqueous species have not yet been obtained or published. Therefore, in this study, they are not taken into account. If Au(HS)2- and AuCl2- are predominant among Au aqueous species and AgCl2- is predominant among Ag aqueous species, the following reactions can be used to derive the relationship among the Ag/Au ratio of electrum and some physicochemical variables (temperature, ∑Au/∑Ag, aH2S, aCl-, pH). Au(HS)2- + (Ag) + 2Cl- + 2H+ = AgCl2- + (Au) + 2H2S (1) in which (Ag) and (Au) represent Ag and Au components in electrum, respectively. From equilibrium relations for reactions (1) and (2), aAg/aAu (in which aAg and aAu denote activities of Ag component and Au component in electrum, respectively) is represented by aAg/aAu = (aAgCl2-•a2H2S)/(K1•aAu(HS)2-•a2Cl-•a2H+) (3) in which K1 is equilibrium constant of (1). aAg/aAu = aAgCl2-/K2•aAuCl2- (4) in which K2 is equilibrium constant of (2). If Au(HS)2- and AgCl2- are predominant Au and Ag species, the equation (3) can be approximated aAg/aAu = (∑Ag•a2H2S)(K1•∑Au•a2Cl-) (5) in which ∑Au and ∑Ag represent total dissolved Au and Ag concentrations, respectively, and γ denotes activity coefficient.If AuCl2- and AgCl2- are predominant Au and Ag species, the equation (4) can be approximated aAg/aAu = (∑Ag)(K2•∑Au) (6) To derive the equations (5) and (6), it is assumed that activity coefficient ratios, γAgCl2-/γAuCl2-, γAgCl2-/γAu(HS)2-, are unity. It is evident in the equations (5) and (6) that aAg/aAu is represented as a function of temperature, aH2S, aCl-, pH, and ∑Ag/∑Au. It can he obtained that aH2S is represented as a function of aS2, aO2, aH2O, and temperature from the following reaction: H2S + 1/2O2 = 1/2S2 + H2O (7) Therefore, in the equilibrium relation for the reaction (7) and equation (5), it is evident that aH2O, aS2, and aO2 also relate to aAg/aAu. It is likely that the activity of H2O liquid generally does not deviate significantly from unity under the hydrothermal condition of concern, considering the salinity of orc-forming fluids estimated from fluid inclusion studies and the relationship betwcen salinity and the activity of H2O liquid (Helgeson, 1969). Therefore, it is reasonable to consider that aH2O is not an important factor controlling aAg/aAu. If electrum is in equilibrium with Ag minerals such as argentite, hessite, and naumannite, the following reactions must be taken into account. 2(Ag) + 1/2S2 = Ag2S (8) From these reactions, it is obvious that aAg/aAu relates to aS2, aSe2, and aTe2. The aS2, aSe2, and aTe2 are represented as a function of ∑S, ∑Se, ∑Te, pH, ionic strength, and temperature. If electrum is in equilibrium with the other Ag minerals (Ag-As, Ag-Sb, Ag-Bi), aAg/aAu depends on concentrations of As, Sb, and Bi in ore-forming fluids. The relationship among asa, temperature, and mole fraction of Ag in electrum has been experimentally studied by Barton and Toulmin (1964) and Barton (1980). Based on the relationship they obtained, Shikazono (1985b) estimated temperature of formation for the epithermal Au-Ag vein-type deposits in Japan and obtained a good correlation of this temperature with a homogenization temperature of fluid inclusions. From reactions (8), (9), and (10), it is obvious that the Ag content of electrum depends on the kinds of Ag minerals. The influences of physicochemical variables (temperature, aH2S, pH, aCl-, aS2, aSe2, aTe2) and mineral assemblage involving Ag minerals on the Ag/Au ratio of electruin are considered below. 2) TemperatureBased on the equations (3) and (4), the relationship between aAg/aAu and temperature was derived, assuming that the values for aH2S, aK+, pH, and ∑Au/∑Ag are constant (Shikazono and Shimizu, 1987). It is also assumed that aK+/aH+ is controlled by K-feldspar/ K-mica/quartz assemblage because this assemblage is common in the Au-Ag deposits studied. Shikazono and Shimizu (1987) indicated that (1) the effect of temperature on aAg/aAu is notably large in Au(HS)2- and AgCl2- dominant solutions and that (2) an incrcase in tcmperaturc causes a decrease in aAg/aAu in Au(HS)2- and AgCl2- dominant solutions. In Fig. 5, the Ag/Au ratios of electrum and homogcnization temperatures for each deposit were summarized. It can be scen in Fig. 5 that the Ag content of electrum in a low temperature region is higher than the content in a high temperature region. There arc two interpretations on this relationship between the Ag content of electrum and temperature: (1) the NAg/NAu of electrum increases with decreasing temperature, even if the chemistry of the solution docs not change, and (2) the NAg/NAu of electrum in the lower temperaturc region is higher than that of electrum in higher temperature region, reflected by the change in dominant Au species; Au(HS)2- is dominant in lower temperature region; and AuCl2- is dominant in higher temperature region. The Ag content and homogenization temperatures plotted in Fig. 5 and other evidences (e.g., ace Fig. 6) may support second possibility. However, more precise thermochemical data on the Au species are clearly necessary to evaluatc the effect of temperature. Especially, thermochemical data on Au chloride complexes and Ag bisulfide complexes must be obtained.
It seems very likely that the concentration of Ag(HS)2- in hydrothermal solutions may be high in low temperature (less than about 300°C) and low salinity fluids, compared with Ag chloride complexes (Barncs, 1978; Henley, 1985; Brown, 1986). When it is, the following reaction has to be considered: Ag(HS)2- + Au = Au(HS)2- + Ag (11) Reaction (11) indicates that the Ag/Au ratio of electrum depends on temperature and ∑Ag/∑Au in Ag(HS)2- and Au(HS)2- dominant solutions. Henley (1985) and Brown (1986) suggested that Ag bisulfide complex is important for controlling Ag concentration in low salinity (probably less than 0.1 weight % chloride concentration) and near neutral geothermal water, such as Broadlands geothermal water (New Zealand). If their suggestion is correct, the Ag/Au ratio of low salinity geothermal water is controlled by the reaction (11).M 3) SalinityDifferentiating the equation (3) with respect to aCl- we obtain ∂[log(aAg/aAu)]/∂(log aCl-) = -2 (12) Therefore, in the Au(HS)2- and AgCl2- dominant region, the Ag content of electrum decreases with increasing aCl- where other variables are constant. However, it is inferred that dominant aqueous Au and Ag species change with the changing of aCl-. For instance, with increasing aCl-, Au(HS)2- may change to AuCl2-, and AgCl may change to AgCl2- with increasing aCl-. In the AuCl2- and AgCl2- dominant regions, aAg/aAu does not depend on aCl-. In the natural chloride-rich hydrothermal solutions, mCl- is related to pH (Shikazono, 1978a). Shikazono (1978a) has derived the relationship between mCl- and pH, assuming the equilibrium among common alteration minerals (quartz, K-mica, K-feldspar, chlorite, albite) and chloride-rich hydrothermal solution. In the region in which monovalent cation (alkali element) concentration exceeds divalent cation (alkali earth element) concentration, pH is related to mCl- (in logarithmic unit) with a slope of approximately -1 on a pH-log mCl- diagram (Shikazono, 1978a). In a divalent cation (alkali earth element) dominant region, the slope is approximately -2. Because of this relationship between pH and mCl-, it is derived that the slope of log (aAg/aAu) vs. log aCl- is not -2, when pH is represented as a function of aCl-. The relationship between pH and mCl- is derived, based on the equilibrium between alteration minerals and hydrothermal solution, However, there are several important processes causing the deviation from the mineral/water equilibrium (mixing, boiling, conductive cooling of fluids; Giggenbach, 1981; Shikazono, 1985c). Figure 6 summarizes the relationship between the Ag content of electrum and the salinity (NaCl equivalent concentration) of fluid inclusions. It can be seen in Fig. 6 that the Ag content of electrum precipitated from lower salinity fluids is higher than that precipitated from higher salinity fluids. For instance, epithermal ore-forming fluids have low salinity, but Kuroko, hypo/mesothermal and skarn. ore-forming fluids have high salinity, and Ag contents of electrum from these deposits are lower than those from epithermal deposits, This observed evidence seems consistent with the theoretical prediction discussed above. Therefore, it is most likely that the Ag content of electrum is controlled by a chloride concentration in hydrothermal solution.
4) pHIt is expected from equation (3) that the Ag content of electrum decreases with decreasing pH of the solution in which dominant Au and Ag species are Au(HS)2- and AgCl2-. The concentration ratio of mΛu (HS)a-/mAuCla- is determined by the equilibrium relation for the following reaction: Au(HS)2- + 2Cl- + 2H+ = AuCl2- + 2H2S (13) From the equilibrium relation for reaction (13), it is derived that mΛuCla-/mΛu(IIS)a- increases with decreasing pH. Thus, at lower pH condition, AuCl2- concentration becomes higher than Au(HS)2- concentration. In AuCl2- and AgCl2- dominant conditions, the Ag/Au ratio of electrum does not depend on pH. In epithermal Au disseminated-type deposits in Japan (Kasuga, Iwato), alteration minerals such as kaolinite, sericite, alunite, and pyrophyllite are commonly observed, and the Ag/Au ratio of electrum is very low (Urashima et al., 1981a). In the Chinkuashih and Chiufen deposits in Taiwan, advanced argillic and argillic alterations, including kaolinite, alunite, and sericite, are associated with Au-Ag mineralization. The Ag content of electrum from these deposits ranges from 31.2 to 44.0 atomic %, which is usually lower than for typical epithermal Au-Ag vein-type deposits in Japan and the Korean Peninsula. Adularia occurs commonly in epithermal Au-Ag vein-type deposits in Japan. Sericite is common in Kuroko-type deposits and hypo/mesothermal vein-type deposits in the Korean. Peninsula (Kaneda et al., 1986). At constant aK+, aSiO2, and temperature, sericite is stable under a lower pH condition than. that for K-feldspar, In general, the Ag content of electrum from Kuroko-type and hypo/mesothermal vein-type deposits is lower than that from epithermal Au-Ag vein-type deposits. Therefore, the relation between the kinds of gangue and alteration minerals and the Ag content of electrum seems to be consistent with the theoretical consideration mentioned above. This suggests that pH is an important factor in determining the Ag content of electrum. 5) Activities of H2S, S2, and O2From equation (3) it is inferred that aAg/aAu increases with increasing aH2S. However, an evaluation of influence of aH2S on the aAg/aAu ratio is difficult because of the difficulty of an estimate of aH2S in ore-forming fluids. The relationship among aS2, aO2, and aAg/aAu in Au(HS)2- and AgCl2- dominant ore-forming fluids can be obtained from equation (3) and the equilibrium relation for the following reaction. H2S + 1/2O2 = 1/2S2 + H2O (14) In Fig. 7, the Ag content of electrum and the FeS content of sphalerite from several types of deposits are summarized. Figures 7-A, 7-B, and 7-C reveal that epithermal Au-Ag vein-type deposits are characterized by a high Ag content of electrum, and a low FeS content of sphalerite and that hypo/mesothermal vein-type deposits and skarn-type deposits are characterized by a low Ag content and a high FeS content. The FeS content of aphalerite from Kuroko-type deposits is low, less than 1 mole % (Shikazono, 1974c), and the Ag content is variable. The FeS content of sphalerite coexisting with iron minerals (pyrite and pyrrhotite) relates to aS2, (Barton and Toulmin, 1966). Thus, it is likely that aS2 for hypo/mesothermal vein-type deposits is different from that for epithermal Au-Ag vein-type deposits (Shikazono and Shimizu, 1987). Estimates of aS2 and temperature for epithermal and hypo/mesothermal vein-type deposits are carried out in Chapter VI. A negative correlation between the FeS content of sphalerite and the Ag content of electrum as shown in Fig. 7 can be explained by the Ag content of electrum from hypo/mesothermal vein-type and skarn-type deposits not being controlled by the equilibrium relation for reaction (8); electrum from these deposits does not coexist with argentite.
6) Minerals Coexisting with ElectrumFigure 8 illustrates the relationship between the Ag content of electrum and the Ag-Au total production ratio from an individual deposit. Shikazono (1986) noted that a positive correlation between the total production ratio of Ag to Au and the Ag-Au ratio of electrum in a given ore deposit seems to exist. The epithermal base metal-rich deposits have a high Ag-Au total production ratio and a high Ag-Au ratio of electrum. In contrast, Au-Ag-rich and base metal poor epithermal deposits have a relatively low Ag content of electrum and a low Ag-Au total production ratio.
It is worthy to note that argentite occurs abundantly in the ore deposits with a high Ag-Au total production ratio, and argentite is poor or absent in the ore deposits having a low Ag-Au total production ratio. This implies that if argentite precipitates in deposits, the Ag content of electrum coprecipitating with argentite is high and the Ag-Au total production ratio of deposits becomes higher. In the deposits with low Ag-Au total production ratio, dominant Ag minerals are Ag sulfosalts (polybasite and pyrargyrite), Ag-Se-S minerals (naumannite, aguilarite, Se-bearing argentite), hessite, and Ag-bearing tetrahedrite. The amounts of these Ag minerals precipitated in a given ore deposit are usually small. Figure 9 summarizes the Ag content of electrum from Cu-rich and Cu-poor deposits. It can be seen in Fig. 9 that the Ag content of electrum from Cu-rich deposits is lower than that from Cu-poor (Pb-Zn-rich) deposits. This relation exists in Kuroko-type and epithermal base metal vein-type deposits. The Ag content of electrum from the siliceous and yellow ores of Kuroko-type deposits in which Cu is rich is lower than that from the black ore in which Pb and Zn are rich but Cu is poor. The dominant Cu minerals in these deposits are chalcopyrite and bornite. Although Cu is not produced from the sulfide-poor deposits (epithermal Au disseminated-type, alaskite-type, and hypo/mesothermal Au veintype deposits), Cu minerals (chalcopyrite, enargite, luzonite) are occasionally found in them, but Pb, Zn, and Ag minerals are poor.
Table 9 shows that the Ag content of electrum coexisting with Bi and/or Te-bearing minerals is relatively low. The main Bi-Te-bearing minerals are hessite and petzite in epithermal Au-Ag vein-type deposits, and hessite, tellurobismuthite, tetradymite, and matildite in hypo/mesothermal vein-type deposits.
It is evident in Table 10 that the Ag content of electrum coexisting with pyrrhotite and arsenopyrite is relatively low. The types of deposits in which pyrrhotite and arsenopyrite commonly occur are hypo/mesothermal vein-type, skarn-type, and alaskite-type deposits. In the other types of deposits, these minerals are rare. The main As-bearing minerals in the other types are tctrahedrite-tennantite (Kuroko and epithermal Au-Ag vein-type) and luzonite and enargite (epithermal Au disseminated-type).
By comparing Figs. 10 and 11 with Table 9 the Ag content of electrum coexisting with argentite, naumannite, and aguilaritc is seen to be highcr than that of electrum coexisting with hessite. Therefore, a difference in Ag minerals coexisting with electrum relates to different Ag contents of electrum, although the reason is still uncertain.
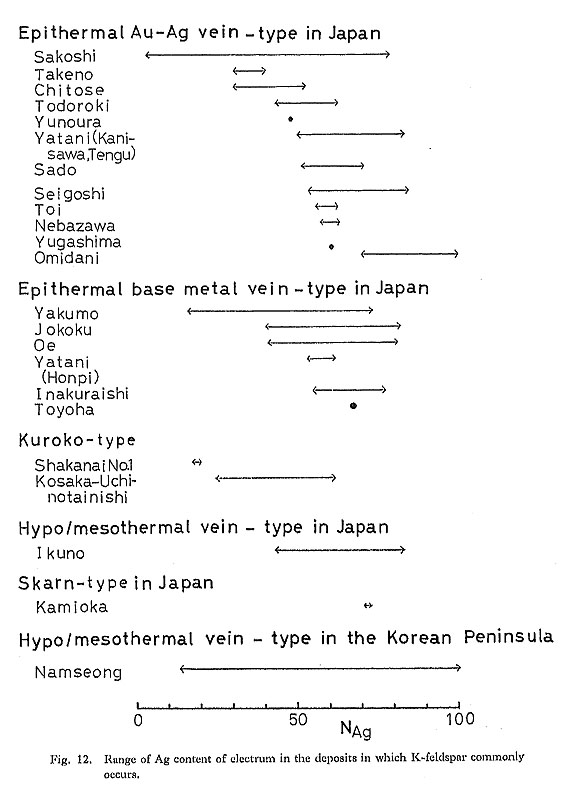
Figure 12 shows the Ag content of electrum from the deposits in which K-feldspar commonly occurs. In general, the Ag content of electrum is high, more than 40 atomic %, On the other hand, the Ag content of clectrum from the deposits in which sericite (muscovite) is common is low (Fig. 13), compared with the Ag content from deposits in which K-feldspar occurs.
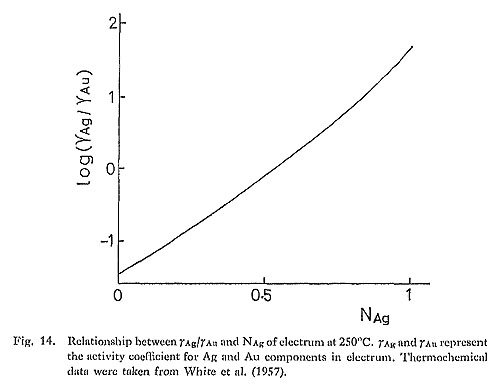
7) Thermochemical Mixing PropertiesTo apply equations (3) and (4) to natural electrum, the activity-composition relationship in Au-Ag alloy must be known. The thermochemical mixing properties of Au-Ag alloy have been studied by White et al. (1957). Based on this study, Barton and Toulmin (1964) successfully derived aS2-temperature-NAg relation in an electrum-argentitc-S2 vapor system, Figure 14 shows the variations in γAg/γAu (γAg: activity coefficient of Ag, γAu: activity coefficient of Au) as a function of NAg calculated from the thermochemical data by White et al, (1957). It can be seen in Fig. 14 that γAg/γAu is close to unity at NAg=0.5, but this ratio deviates significantly from unity toward higher and also lower NAg valucs.
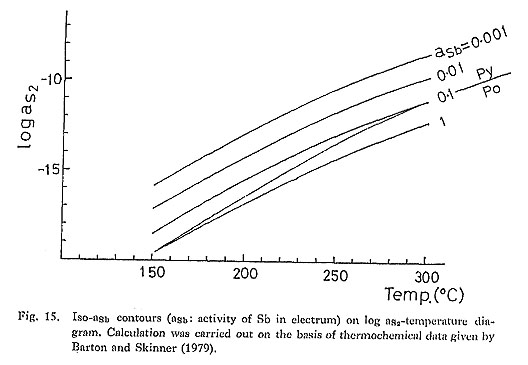
2. Contents of Other ElementsHg content:If electrum is in equilibrium with cinnabar, the following reaction can be used to obtain the relationship among the Hg content of electrum, asa and temperature. (Hg) + 1/2S2 = HgS (15) in which (Hg) is the Hg component in electrum. From the equilibrium for (15), it is obvious that aHg is represented as a function of temperature and aS2 and that aHg increases with decreasing aS2 at a given temperature, Shikazono and Shimizu (1988) have reported a high Hg content of electrum from the Tsugu Au-Sb-Hg vein-type deposit (Hg: up to 7.7 atomic %) and indicated that electrum having a high Hg content occurs in Au deposits in which the opaque minerals indicating low aS2 conditions (pyrrhotite, cubanite) occur. This occurrence of opaque minerals is consistent with a high Hg content of electrum from. these deposits. If HgS is not present, aS2 for electrum depends on the chemical states of Hg in oreforming fluids. In this case, aHg depends on the factors (e.g., ∑Hg in ore-forming fluids) other than aS2 and temperature. There are several possible Hg species in orc-fornaing fluids (e.g., Hg bisulfide complex and organic complexes) and a transport of Hg by vapor is also probable (Barnes, 1978). Cu content:The Cu content of electrum coexisting with copper and iron minerals (chalcocite, chalcopyrite, pyrite, pyrrhotite) is determined by the following reactions. 2(Cu) + 1/2S2 = Cu2S (16) in which (Cu) is the Cu component in electrum. The reactions (16) to (20) indicate that aCu (activity of Cu component in electrum) epends only on temperature in the pyrite and chalcopyrite stable region, whereas it depends on aS2 and temperature in the bornite, chalcocite, covellite, and pyrrhotite stable regions. In these regions, aCu increases with decreasing aS2 at a given temperature. Yamaoka (1981) reported high Cu content of electrum from the Kamaishi skarn deposit, Cuprian electruni from the Kamaishi deposit coexists with pyrrhotite. Thus, it is inferred that cuprian gold formed under a relatively low aS2 condition. However, data on cuprian eleetrum) are scarce, and a mode of occurrence of cuprian electrum is not well known. Further works on the Cu content of electrum arc required. Sb content:The Sb content of electrum in equilibrium with stibnite is controlled by the following reaction: 2(Sb) + 3/2S2 = Sb2S3 (21) in which (Sb) is the Sb component in electrum. Reaction (21) implies that aSb increases with decreasing aS2 at a given temperature (Fig. 15). Under the low aS2 condition, gold reacts with stibnite to form aurostibite. It was reported by Urashima et al. (1981) that the Sb content of electrum from the Sakoshi-Odomari deposit is high (up to 2.4 weight %). The activity of S2 for the Sakoshi-Odomari mineralization is estimated to be relatively low because pyrrhotite is found and the FeS content of sphalerite is high (5.6 to 7.9 atomic % Fe; Urashima et al., 1981b), compared with the typical Fe content of sphalerite from epithermal Au-Ag vein-type deposits (Shikazono, 1977b). Low aS2 condition is consistent with high Sb content of electrum from the Sakoshi-Odomari deposit. The thermochemical mixing properties of Au-Ag-Sb alloy must bc studied to estimate the aS2-temperature range from the Sb content of electrum.
|