CHAPTER V
Important Food Resources for Reef Fishes
|
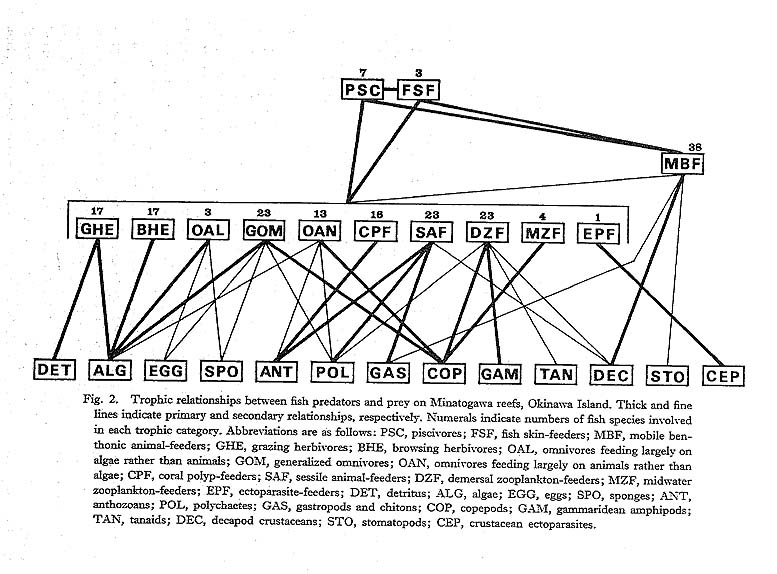
The trophic relationships between fish predators and prey on Minatogawa reefs are summarized in Fig. 2. There is no doubt that various algae are one of the most important food resources for reef fishes. All predators among the four trophic categories utilize algae as primary food by grazing or browsing on benthonic algae or picking drifting algal fragments. The standing crop or primary production of benthonic algae on coral reefs has been recognized as being extremely high. For example, Bakus (1967) reported that the production of blue-green algae on the outer reef at Eniwetok Island may occasionally be over 200 times more than that of littoral micro-algae from sand and flats in Long Island Sound (41°N), Furthermore, he estimated that the productivity of bluegreen algae for a reef flat of about 65,000 m2 at the island is roughly 520,000 k-cal/day, and that the energy needed to support the standing crop of scarid fishes within the reef flat is about 22,000 k-cal/day, leaving a surplus of 498,000 k-cal/day, in part available for other grazers and browsers. He thus concluded that "there is sufficient energy available in the survey area to support the scarid fishes, and apparently enough remaining to support acanthurid fishes and other browsers and grazers." On the other hand, drifting algal fragments are also abundant in midwater above the reefs. Johannes and Gerber (1974) found that algal fragments constituted well over 50% of the total plankton biomass collected in the water over the reefs at Eniwetok. Therefore, many reef fishes were probably adapted for efficiently exploiting these rich energy resources.
Many zooplankton-feeders consume exclusively demersal zooplankters, mostly harpacticoid copepods, gammaridean amphipods, tanaids, and certain polychaetes. Especially, harpacticoid copcpods have been known as a very important food resource for juvenile fishes of several demersal species, including certain pomadasyids, gerreids, and sparids (Albeit and Scheibd, 1982), as well as many adult reef fishea. As listed in Table 3, the midwater zooplankton-feeders are very rare, with only four species as compared to twenty-three demersal zooplankton-feeders. This phenomenon may be explained in terms of the relative abundance of both types of prey. That is, although we did not investigate their abundance on Minatogawa reefs, Alldredge and King (1977) reported that the density of demersal zooplankters in the Lizard Island Lagoon, Great Barrier Reef, is about ten times higher than that of midwater zooplankters. The demersal zooplankton-feeders demonstrated at least two different feeding strategies: One is diurnal feeding and the other is nocturnal feeding. Some diurnal predators, for instance labrids, habitually hover close to the reef, inspecting its surface, and seize demersal zooplankters that are concealed in shelters during the day. On the other hand some nocturnal apogonids heavily hunt their specific prey that emerge in the exposed water column above the reefs after dark from shelters. In addition to these predators, utilization of demersal zooplankters as primary food is also recognized among several omnivorous predators at Minato gawa.
Scleractinian corals, both polyps and mucus, seem to be significant prey for reef fishes, although coelenterates are not utilized as food for fishes in most other marine communities (Hiatt and Strasburg, 1960). On Minatogawa reefs, sixteen species feed predominantly on only living corals (Table 3), and also some other predators, especially certain chaetodontids, prey on them at times. These predators have highly specialized feeding mechanisms to feed on coral polyps which are firmly hemmed around by calcareous armor (Hiatt and Strasburg, 1960; Hobson, 1974, 1975). For instance, the monacanthid Oxymonacanthus longirostris and some chactodontids possess extended snouts and small teeth protruding from their mouths with which they bite off the tips of individual polyps that come slightly out of the armor. The bleniid Exallias brevis actively scrapes off the surfaces of living corals with its comb-like teeth. The tetraodontid Telraodon nigropunctatus breaks off living tips of branching corals with its fused, beak-like teeth. On the other hand, coral mucus is also used as food by reef animals other than fishes, Johannes (1967) suggested that suspended particulate organic aggregates consisting of coral mucus may be an important food resource for zooplankters in the vicinity of coral reefs. Furthermore, Knudsen (1967) and Patton (1974) reported that decapod crustaceans such as Trapezia species commensal with corals feed primarilyon coral mucus. Undoubtedly, thls evidence indicates that scleractinian corals arc an important contributor to food chains of coral reef ecosystems. Finally, benthonic invertebrates such as decapod crustaceans, sponges, sea anemones, gastropods, chitons, and polychaetes arc among the most available food for carnivorous and omnivorous fishes, Decapod crustaceans are especially heavily utilized as primary food by many predators, e.g., mobile benthonic animal-feeders (Fig. 2). These benthonic prey have various specific defensive structures such as shells, nematocysts, or spines, or secretive habits. Nevertheless, benthos-feeding fishes successfully take such prey by highly adaptive, multifarious feeding mechanisms. This phenomenon has been considered to be a result of coevolution between prey and predators. Hobson (1974) noted that "predation pressures lead to defensive adjustments in prey and these in turn stimulate further offensive modifications in predators," |