CHAPTER VI
Mineralogy of the Tokuwa Batholith Rocks
1) Amphibole
|
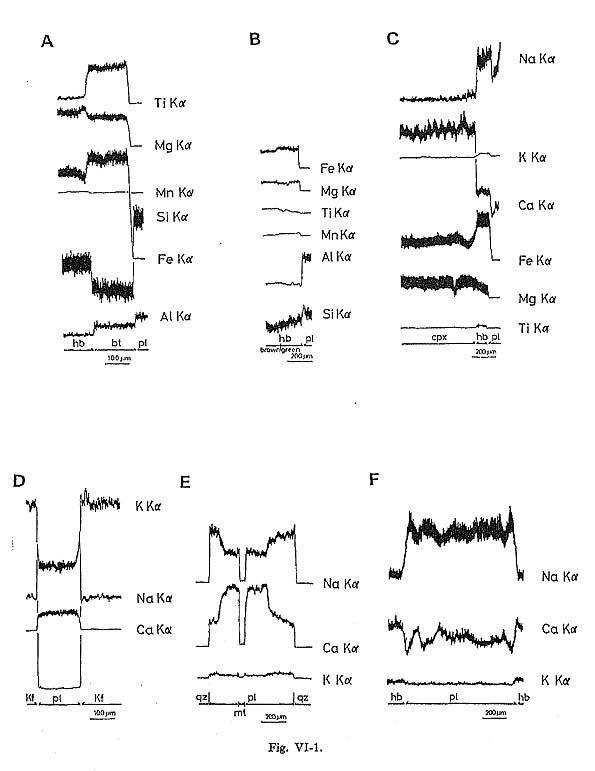
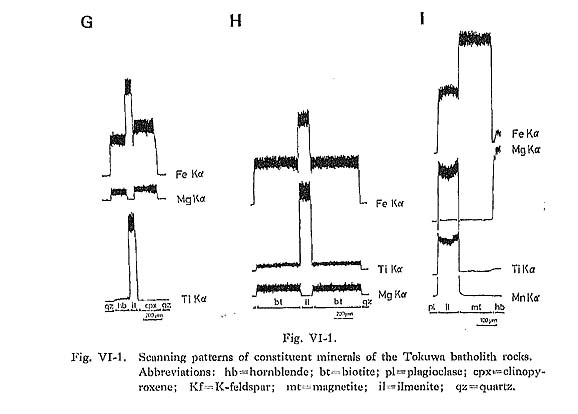
Hornblende commonly occurs as a euhedral to subhedral prismatic crystal with a maximum length of 2 mm. It occasionally contains pyroxene and cummingtonite in rocks from the central parts of the batholith. In the core, hornblende containing pyroxene is found exclusively in rocks of gabbroic to dioritic composition. Plagioclase is occasionally found in hornblende grains. The pleochroism of hornblende in magnetite-series rocks (yellowish green) is distinctly darker than that of hornblende in ilmenite-series rocks (greenish yellow). Hornblende in magnetite-series rocks comprises the following: X': gray yellow to pale greenish yellow or moderate yellowish green; Hornblende in ilmenite-series rocks is divided into: X': pale greenish yellow or grayish yellow; Occasionally a weak zonal pattern is seen (Figs. VI-1-A, -B, -C, and -F and Plate 11): the greenish rim is enriched in SiO2, FeO* (total Fe as FeO) and MnO, and the brownish core in TiO2, Al2O3, MgO, Na2O, and K2O (Table VI-1). However, the rim is enriched in MgO when hornblende is in contact with biotite (Fig. VI-1-A).
Fifty representative chemical data on amphibole from the Tokuwa batholith rocks are shown in Table VI-2. Calculation of hornblende Fe2O3 contents was based on the method proposed by Papike et al. (1974): Fe2O3 contents were calculated from the charge balance equation, Fe3+ = AlIV + NaB - (Na, K)A - AlVI - 2Ti4+, contents of all oxides were normalized based on 23 oxygens, and new Fe2O3 contents were calculated until there was no change in the derived Fe3+/Fe2+ ratio. Hornblende from the batholithic rocks is generally identified as magnesio-hornblende, ferro-hornblende, actinolitic hornblende, and ferroactinolitic hornblende with small amounts of actinolite and ferroactinolite, according to the nomenclature of Leake (1978) (Fig. VI-2). Figure VI-2 also shows that the mg values (Mg/(Mg+Fe)) of hornblende in magnetite-series rocks (0.44 to 0.73; mean 0.55) are slightly but significantly higher than those of hornblende in ilmenite-series rocks (0.42 to 0.60; mean 0.49). This may be due either to consumption of Fe to form Fe-Ti oxide minerals in magnetite-series rocks or to the effect of oxygen fugacity on Fe partitioning between the magma and hornblende. The Si content (number of Si atoms in the unit cell formula of amphibole) of hornblende in magnetite-series rocks (6.59 to 7.37) is practically identical to that of hornblende in ilmenite-series rocks (6.63 to 7.79). Hornblende in magnetiteseries rocks (0.420 to 0.896; mean 0.674) is lower in atomic Fe2++Fe3+) ratios than hornblende in ilmenite-series rocks (0.541 to 0.926; mean 0.711). High tetrahedral Al (AlIV) contents are generally considered to be essential to balance the associated high Fe3+ contents in order to maintain a charge balance between the octahedral and tetrahedral sites. The analytical data (Table VI-2) on hornblende in the Tokuwa batholith rocks were examined using a series of correlation diagrams between paired constituents. Relatively significant correlations were observed between: (Na+K) and AlIV
Many possible substitutions control amphibole chemistry, but the most common types of amphibole solid solution can be represented by the general formula (Leake, 1978): A0-1B2CVI5TIV8O22(OH, F, Cl)2 where A = Na, K, (vacancy): A site; Robinson et al. (1971) and Czamanske et al. (1977) showed that there are at least six possible schemes of coupled substitutions which involve the charge balance within the amphibole structure, in addition to simple substitutions, such as Fe2+ for Mg.
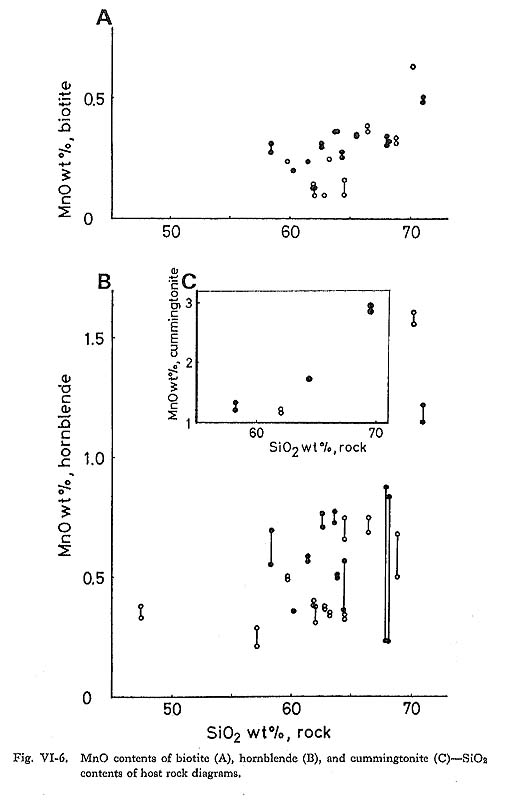
It is concluded from the observed relations that at least coupled substitution mechanisms 1, 2(a), 2(b), 3(a), 3(b), and 5 above are operative in the hornblende of the Tokuwa batholith rocks. In particular, the presence of coupled substitutions 1 and 5 is demonstrated clearly in the correlation diagrams (Figs. VI-4-A and -C). Upon examing the relations between the bulk chemical compositions of the rocks and the corresponding hornblende compositions, the following were found: (1) The mg values of hornblende in magnetite-series rocks of the Tokuwa batholith decrease as the SiO2 content of the host rocks increase, whereas the mg values of hornblende in ilmenite-series rocks show a rather narrow range of variation (Fig. VI-5-A). Czamanske et at (1981) reported that mg values of hornblende in magnetite-series granitoids of the Daito and Yokota granitic bodies decrease with the increase in SiO2 of the host rocks. This was not found in the present study. (2) MnO contents of hornblende increase as SiO2 contents of host rocks increase (Fig. VI-6-B).
Cummingtonite occasionally occurs as an aggregate of several subhedral grains up to 0.4 mm long at the rim of hornblende crystals. The MnO contents of cummingtonite increase with the increase in SiO2 of host rocks (Fie. VI-6-C). | |||||||||||||||||||||||||||||||||