General Features of Cave Bivalves
|
The bivalve fauna discovered in several sublittoral limestone caves of the Ryukyu Islands is unique in many respects. As described in the systematic part, it consists of 48 species belonging to 36 genera in total (Table 1). Some of them belong to groups unfamiliar to the shallow-water faunas of tropical-subtropical Indo-West Pacific realm. In this section we summarize the common characteristics of this fauna and discuss its taxonomic, ecological and evolutionary significance.
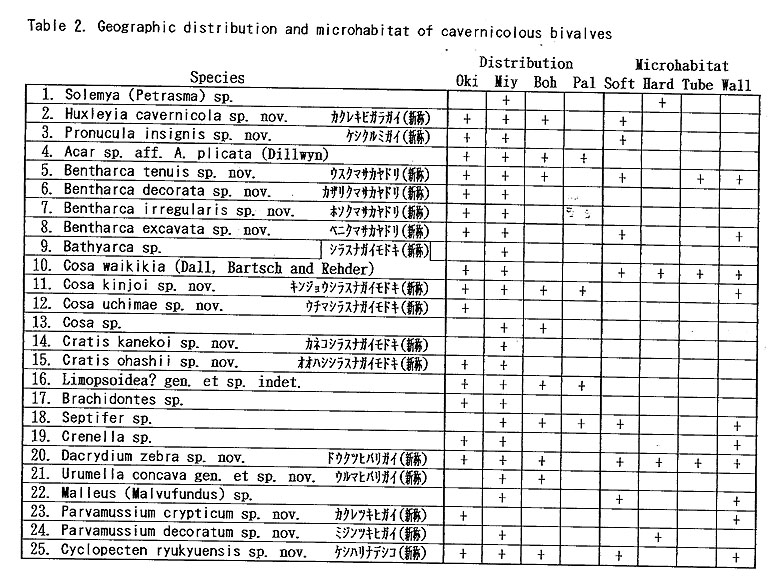
As listed preliminarily (Kase and Hayami, 1992), 35 bivalves were distinguished in the samples collected during 1990-1991 from two submarine caves ("Shodokutsu" and "Daidokutsu") of Ie Islet, Okinawa. The following common characteristics were emphasized in our preliminary study: 1) very small adult shell size (usually less than 5 mm in length), 2) mostly colorless or translucent shells, 3) mostly epifaunal or semiinfaunal suspension feeders, 4) absence of protobranch species except for a nucinellid, 5) many bathyal and abyssal affinities, 6) persistent denticles of provinculum retained until adult stage in many pteriomorph species, suggesting significant paedomorphosis by progenesis, 7) unusually large (sometimes hat-shaped) prodissoconch I in many species, suggesting non-planktotrophic development. Most of these characteristics are also recognized in the subsequently examined bivalves from several similar sublittoral caves of Shimoji and Irabu Islets, Miyako Islands, though the assemblage as well as the relative frequency of common species is somewhat different from that of Ie Islet (Table 2).
The unique assemblage of these bivalves may be related to various physico-chemical and biological factors peculiar to such sheltered environments. Available data about cavernicolous organisms other than molluscs are still limited, but the analyses of the composition, species diversity, stunting, heterochrony, archaism and developmental strategy of these bivalves, which can be based on the collected samples, may be important for consideration of the evolutionary significance of this cryptic fauna. 1. Recognition of cryptic bivalvesIt is a fundamental question whether or not these bivalves are really indigenous to such sheltered environments. Some scientists have doubted the endemism of these bivalves which was stressed in our preliminary reports. Because non-cryptic bivalves of such diminutive size have not been sufficiently investigated in Japan and its adjacent regions, we cannot necessarily give an obvious answer to this question. In fact, abraded shells of Cosa waikikia, Gratis kanekoi, Gratis ohashii, Chlamydella incubata, Rochefortina sandwichensis and Hiatella sp. aff. H. orientalis have been obtained from dredged or beach sands of Okinawa and some other regions of southern and central Japan (Kaneko, 1984, 1991; our unpublished data). Little, however, has been known about the actual microhabitat of these species outside the caves. The following species are actually represented by a large number of living specimens, and it is obvious that, if not strictly endemic, they inhabit cavernicolous environments by preference: Huxleyia cavernicola, Pronucula insignis, Bentharca tenuis, Bentharca excavata, Cosa waikikia, Dacrydium zebra, Urumella concava, Parvamussium crypticum, Cyclopecten ryukyuensis, Chlamydella incubata, Chlamydella tenuistriata, Pycnodonte tanigiichii, Limatula kinjoi, Epicodakia pygmaea, Carditella iejimensis, Carditella shimojiensis, Salapntium unicum, Rochefortina sandwichensis and Coralliophaga hyalina. As shown in Table 2, these bivalves were found alive on the sediment surface, rock fragments, polychaete tubes and/or cave walls. The following species are also regarded as cryptic because numerous empty shells occur exclusively in the cave sediments: Bentharca decorata, Cosa kinjoi, Cosa uchimae, Limopsoidea gen. and sp. indet., Divarilima elegans and Halonympha asiatica. Possibilities for ecophenotypic effect and concentration of immature individuals must also be examined. In many cases, however, asymptotic size distribution indicating the ultimate adult size and other lines of evidence (e.g. parental care of embryos and commarginal lamellae becoming denser near the ventral margin) seem to deny the possibility of invalid dispersal of juveniles. Incubation of several embryos is actually observed in many specimens of Cosa waikikia, Dacrydium zebra, Chlamydella incubata and Chlamydella tenuissima, which were collected in late June of 1992 and mid April of 1993. Only a few living individuals of Solemya (Petrasma) sp., Septifer sp., Crenella sp., Lima sp., Limaria sp. and Irus 2 spp. were found in the caves. Acar sp. aff. A. plicata, Bathyarca sp., Brachidontes sp., Cardita sp, and Hiatella sp. aff. H, orientalis are represented by a small number of empty shells from the cave sediments. Because the adult shell size is still difficult to estimate from the cave specimens, it is uncertain whether these species are actually reproducing in such cavernicolous environments. They may also be undescribed stunted species, but we hesitate to propose new specific names for this reason. 2. Biased composition of taxonomic groupsThe Arcoidea, Limopsoidea, Mytiloidea, Pectinoidea, Limoidea and Carditoidea are the most dominant bivalve superfamilies in these caves, not only in species diversity but also in number of individuals. These superfamilies are also common in exposed environments of the same region, but their familial, subfamilial and generic compositions are unique. The cave Arcoidea are mostly composed of species belonging to Bentharca, and the Limopsoidea belong to Cosa and Gratis of the Philobryidae. All the cave Pectinoidea belong to the Propeamussiidae (Parvamussium, Cyclopecten and Chlamydella) instead of to the Pectinidae, Plicatulidae and Spondylidae. The abundant occurrence of the species belonging to Huxleyia, Dacrydium, Divarilima, Carditella and Halonympha is also remarkable, because these genera scarcely occur in the upper sublittoral faunas of this region. In contrast, the absence or rarity of the species belonging to the Pterioidea, Anomioidea, Ostreoidea, Chamoidea, Cardioidea, Mactroidea, Tellinoidea, and Veneroidea (except some cavity-dwelling genera) is also noticed, because these superfamilies generally exhibit large species diversity in and around the coral reefs of this region. Pycnodonte taniguchii is a solitary cementing bivalve, but its habitat is restricted to the gloomy walls and ceilings near cave entrances and beneath overhangs (Hayami and Kase, 1992), Malleus (Malvufundus) sp. characteristically inhabits some narrow cavities of cave walls, though its stunted and peculiar morphology may only be due to some ecophenotypic effect in a widely distributed species. Another large-sized bivalve, Glossocardia obesa, may also be a cryptic species, because the empty shells (and also living individuals according to divers) have been found primarily from the bottom of such a sheltered environment. Most of the cave bivalves must be epifaunal or semi-infaunal suspension feeders, though a few possible deposit feeders (Solemya, Huxleyia and Promicula) and a carnivorous species of the Cuspidariidae have been discovered in association. Symbioses with sulfate-reducing bacteria have been recognized or inferred in some small-sized species of the Solemyidae and Nucinellidae as well as in many bivalves around deepwater hydrothermal vents (Reid, 1990, etc.). This trophic habit is, however, unlikely for these cave bivalves because the cave sediments are free from sulfur dioxide and never indicate an anaerobic condition. 3. Stunting of the cave bivalvesAll of the cave bivalves, except for Malleus (Malvufundus) sp., Pycnodonte taniguchii and Glossocardia obesa, are very small in adult shell size (generally less than 5 mm in length and height). This is also the case with the associated gastropods and brachiopods. Neritopsis radula and certain soft sponges are the only conspicuous large-sized benthic organisms in the lightless innermost part of these caves. Remarkable stunting, regardless of taxonomic groups, appears to have occurred in the cave fauna. The diminutive shell-size of the present cave bivalves may be the result of two different processes. One is the superficial stunting caused by the arrival of species which belong to originally small-sized taxonomic groups; for example, the cave species of Cosa, Cratis, Dacrydium, Rochefortina and Kelliella are. not much smaller than ordinary non-cryptic species of the same genera. The other is the true stunting which may have occurred in adaptation to the cryptic environment; for example, the cave species of Huxleyia, Promicula, Bentharca, Parvamnssium, Cydopecten, Chlamydella, Divarilima, Ctenoides, Limatula, Cardita, Carditella, Coralliophaga and Halonympha are much smaller than the most closely related non-cryptic species. Several paleontologists reviewed previous studies of "dwarf" or "micromorph" faunas of fossil and extant marine benthic invertebrates and discussed causal factors of stunting (Tasch, 1953; Ager, 1963; Hallam, 1965; Mancini, 1978; etc.). They regarded abnormal salinity, metallic cations (Fe, Cu), oxygen deficiency, high turbidity, strong water agitation, high population density, abnormal pH, temperature variations and deficient food supply as possible causes. One famous example is the stunting of bivalves (e.g. Mytilus edulis and Cardium edule) in the Baltic Sea, where the shell size and the salinity show a significant correlation with each other (Sorgenfrei, 1958). Such a correlation, however, does not necessarily mean direct causal relation. In the present caves, the salinity seems to be normal, notwithstanding that the caves must have been formed by underground water during some low sea-level stages of the Pleistocene. At present, the level of underground water in these islets is almost equal to or a little above the sea level, as evidenced by a number of active limestone caves open to the coastal cliffs. Two tubular sediment cores (approximately 16 and 20 cm in length) were success fully taken by a cooperative diver at the innermost part of the cave "Shodokutsu" of Ie Islet. The sediments are parallel-laminated and scarcely bioturbated. The cores were sliced every 2 cm in height, and the sediments and organic remains in each subsample were examined. The physical environment of this site seems to have been maintained for a long time (probably several thousand years) under a tranquil but never oxygen depleted condition. The water temperature in the caves is almost the same as, or only slightly lower than, that of the outside, at least in the seasons from spring to autumn. In the Ryukyu Islands the temperature of oceanic waters is maintained between 21° and 28° throughout a year. There is also no evidence that any of these cave bivalves is parasitic. Of the above-mentioned possible factors, nutritional deficiency is likely to place a strong constraint on the growth rate and ultimate body size of the present cave bivalves, though some other factors (e.g. selective predation on larger-sized species) may also be related to the stunting. Thiel (1975) and many others regarded the diminutive size of most deep-water benthos as related to limited nutrient supply. Vermeij (1990) presented the interesting hypothesis that a general decline in. nutrients for suspension feeders from the western rim of the tropical Pacific to the oceanic islands results in smaller adult size as well as lower species diversity. As pointed out by Snyder and Bretsky (1971) and Gould (1977: 325), stunting may be a positive response to a particular condition (oligotrophic environment in this case). Examinations of trophic nature, larval development and heterochrony may be important to test this hypothesis, because oligotrophic condition seems to have strong influence on the life cycle and adaptive strategy of organisms. Another question to be examined is the possibility that these diminutive bivalves might be interstitial animals. Sediment surface samples from a cave of Ie Islet often contain numerous living individuals of Carditella iejimensis, and Huxleyia cavernicola, Cosa waikikia, Dacrydium zebra, Chlamydella incubata, Carditella shimojiensis and Rochefortina sandwichensis are found in samples from some caves of Shimoji Islet. These bivalves are mostly regarded as epibyssate or semi-infaunal species. The shells of these living bivalves attain 1 to 3 mm in length and height in the adult stages, and seem to be larger than the sediment particles. Commonly known interstitial meiobenthos are generally smaller than 500 p.m in breadth, So far as we observed in the core samples taken at the innermost part of "Shodokutsu", no bivalve individual seems to live below the sediment surface. In these bivalves, therefore, it is unlikely that the selection for smaller shell-size is related to interstitial life. Moreover, numerous living individuals of Cosa waikikia, Dacrydium zebra and Chlamydella incubata were found attached by the byssus to the terminal part of polychaete tubes (Figure 212) exposed above the sediment surface as well as to sediment grains, rock fragments and cave walls.
4. Species diversity and biomassThe species diversity of bivalves in each cave appears to be quite great. For example, different species within a genus often occur together in the bottom sediments at one and the same locality. A sediment sample at one locality often contains numerous empty shells of more than 30 bivalves. However, the great diversity may sometimes be superficial, because it is not necessarily based on actually living specimens. In spite of our repeated examinations of samples carefully collected from the cave wall and sediment surfaces, living individuals are relatively few in comparison with empty shells. In a cave of Ie Islet ("Shodokutsu"), for example, Bentharca, Cosa, Dacrydium, Parvamussium, Cardita and Carditella was found alive, but each genus is represented by one species. In spite of thorough sampling in this cave, other bivalve species are represented by numerous but dead specimens. More than 90 % of living individuals from the bottom sediments belong to Carditella iejimensis. Therefore, the composition of bivalves in this cave should be regarded as an integration of all the species that ever lived there over many years. Because the inner part of this cave has remained in a lowenergetic physical condition, even delicate molluscan shells can be preserved for a long time without destruction. In spite of the apparent great diversity and large quantity of organic remains, the biomass per unit space is very small. Species diversity of bivalves is also generally low in the totally dark caves of Shimoji Islet. In most caves of Shimoji and Irabu Islets, Cosa waikikia, Dacrydium zebra or Chlamydella incubata constitute the dominant species; more than 90% of living individuals belong to the three species. It is interesting, as will be discussed later, that all three dominant bivalves are brooding species. In a gloomy cave of Irabu Islet (called "Cross Hole"), however, more than 20 bivalves were found alive at one and the same sampling point, where other living organisms and polychaete tubes were also abundant. Other sediment samples in the same cave did not necessarily contain so many living organisms. This fact indicates that the distribution of organisms in each cave is patchy rather than uniform or random. The spectral change of organisms from the entrance to the innermost part of each cave is indeed the most interesting subject of study, but systematic sampling for this purpose is difficult because of the patchy distribution of organisms and the restricted distribution of fine-grained sediments on the cave floor. 5. Affinities with deep-water faunasOne of the most interesting features of the present cave bivalves is the presence of many lower sublittoral, bathyal and abyssal affinities (Table 3). No cave bivalve is strictly identical with deep-water species, but this does not rule out the possibility of direct gene flows between these caves and deep-sea bottoms. Most significant deepwater affinities are the species of Huxleyia, Bentharca, Cratis, Dacrydium, Parvamussium, Chlamydella, Divarilima, Carditella, Kelliella and Halonympha.
Because the sampling sites of the present material lie between sea level and about -40 m, the discovery of these genera certainly breaks the shallowest records of their bathymetric distribution. At the same time it is suggested that neither high hydrostatic pressure nor low water temperature is a decisive factor for the restricted distribution of these genera. Although certain species of Huxleyia, Dacrydium and Parvamussium often inhabit sublittoral substrata in high latitudinal seas (Bernard, 1983, etc.), these genera so far recorded in low-middle latitudinal seas are restricted to bathyal to abyssal depths. Moreover, Bentharca, Dacrydium, Kelliella and Halonympha are important constituent genera in abyssal faunas (Clarke, 1962; Knudsen, 1967, 1970; Oliver and Allen, 1980; Allen and Morgan, 1981; Poutiers, 1989; etc.). Why do so many species of deep-water-type genera inhabit these sublittoral caves? Some investigators have asked why cryptic organisms resemble deep-sea ones (Iliffe, Hart and Manning, 1983; Vermeij, 1987; Kobluk, 1988). Their interpretations are somewhat different, and their discussions were not necessarily based on substantial molluscan data. Iliffe, Hart and Manning (1983) seem to have attributed the occurrence of deep-sea type decapod species in some inland marine caves of Bermuda in part to the small seasonal change of water temperature. This reasoning, however, is hardly applicable for the present cave bivalves, because the water temperature in the caves is not much different from that of the exposed environment. Kobluk (1988) attached importance to the low level of illumination, which gives an analogous constraint to cryptic and deep-sea organisms. On the other hand, Vermeij (1987) emphasized the low level of predation pressure in both cryptic and deep-sea environments. The resemblance between the cave and deep-water bivalve faunas can be recognized not only in their taxonomic aspect but also in their shell-size and developmental strategy. As will be summarized by Allen (1983), the biomass and shell-size of molluscs decrease significantly with depth; abyssal infaunal molluscs are rarely larger than 5 mm. As will be discussed later, the general adaptive strategy of the cave bivalves, as judged from the features of Pd I, is comparable with that of deep-sea and cold-water bivalve faunas. On the other hand, some compositional differences exist between the cave and deep-water bivalve faunas. Deposit feeders and carnivores are decidedly less common in the cave bivalves. The Nuculanoidea, for example, constitute dominant groups in deep-water faunas (Knudsen, 1970; Allen, 1983), but no nuculanacean shell has been found in the sediments of these caves. The Nuculoidea and Poromyoidea are represented by only one species each. No representatives of the Thyasiridae and Pandoroidea have been found. Some cave species belong to such shallow-water genera as Malleus, Coralliophaga, Ims and Hiatella. Significant stunting also seems to have occurred in these species, but their planktotrophic developmental strategy (indicated by the presence of Pd II) is retained. It is noticed that these genera mainly consist of coral-nestling and cavitydwelling species (Morton, 1980, 1983, 1990, etc.), and their occurrence in dark caves may not be very strange. 6. Paedomorphosis by progenesisAs described in the systematic part, paedomorphic features are often observed in these cavernicolous bivalves. In many pteriomorph species, numerous subvertical denticles along the dorsal margin persist until the latest growth stage. We regard them as a paedomorphic retention of provinculum. The relatively small number of adult hinge teeth in the cave species of Huxleyia, Pronucida, Acar, Bentharca and Bathyarca may also be a paedomorphic feature, because they are additional with growth. More than half of these cavernicolous bivalves are regarded as paedomorphic (Table 4).
Two different processes are assumed for the paedomorphosis. One is the adaptation of intrinsically paedomorphic groups to the cryptic environment, Cosa, Gratis and Dacrydium species are regarded as good examples of this case, because all the species of these genera reveal similarly diminutive shell-size and paedomorphic features. As suggested by Boss (1969), Kelliella is probably also a paedomorphic veneroid genus. The other is the case in which rapid paedomorphic evolution possibly occurred in adaptation to the cryptic environment. Most cavernicolous species of the Arcidae, Mytilidae, Propeamussiidae and Limidae retain numerous denticles of provinculum throughout the ontogeny, but at the same time the shell-size is much reduced in comparison with closely related non-cryptic species. In some cave arcids, e.g. Bentharca excavata, primary alivincular amphidetic ligament is active throughout the growth, and an incipient secondary duplivincular ligament appears only occasionally on the posterior cardinal area in adult individuals (Figures 60, 61). Because this feature corresponds to the ligament structure of juvenile arcid shells of similar size, the phenomenon should be regarded as paedomorphosis by progenesis.
The Philobryidae were commonly regarded as a neotenous group derived from the Limopsidae (Tevesz, 1977; Morton, 1978). Many species of the Philobryidae have persistent denticles of provinculum, and prionodont teeth are absent or only incipiently developed even in adult individuals. If the phylogenetic relation is accepted, the paedomorphic evolution is better regarded as due to progenesis instead of neoteny,because significant size reduction has occurred. The shell-size of the Philobryidae is generally one digit smaller than that of the Limopsidae. Considering the much-reduced shell-size in many species of other families, paedomorphosis by progenesis is a widespread phenomenon in the present bivalve fauna. Little is known about the growth rates and life cycles of these cavernicolous bivalves. It is, however, likely that their growth is generally slow, because nutrition is generally deficient in the caves. Several studies have indicated that slow growth in bivalves is associated with strong shell convexity (Seed, 1968; Vermeij, 1990), We intuitively feel that the cave bivalves, especially the species of the Nucinellidae, Arcidae, Phylobryidae, Mytilidae, Propeamussiidae, Limidae, Carditidae and Trapeziidae, generally reveal strong shell convexity in comparison with those of ordinary environments. 7. Dominance of non-planktotrophic developmentThe mode of larval development of molluscs, in general, has been classified into three types: planktotrophic, lecithotrophic, and direct development (Thorson, 1946, 1950). The following relation between the developmental strategy and the egg size in bivalves has been approved (Ockelmann, 1965; Jablonski and Lutz, 1980, 1983; Mackie, 1984): It is interesting that the ratio of developmental types changes geographically. Ockelmann (1965) examined the relative frequency of many shallow-water bivalve faunas along the European Atlantic coast from Gibraltar to Spitzbergen. The ratio of planktotrophic species significantly decreases toward the north; it is about 76 % (N=125) near Gibraltar but only about 15 % (N= 53) in Spitzbergen (Table 5). A similar tendency has been known also in gastropods of this region (Thorson, 1965). This is generally called "Thorson's Rule". Bivalves of non-planktotrophic development are more frequent in deep-waters than in shallow waters (Ockelmann, 1965; Alien, 1983; Mackie, 1984). Knudsen (1967, 1970) suggested that the deep-water molluscs collected by the John Murray Expedition consist of 8 % planktotrophic, 58 % lecithotrophic and 34 % directly developing species, and that those collected by the Galathea Expedition are composed of 9 % planktotrophic, 78 % lecithotrophic and 13 % directly developing species (Table 5). Although the egg size of bivalves seems to decrease from bathyal to abyssal-hadal (Mackie, 1984), the difference of developmental strategy between shallow- and deepwater bivalves is quite obvious, at least in low and middle latitudinal regions.
In the present cave bivalves the prodissoconch is generally well preserved without abrasion (or erosion), probably because of the low-energetic physical condition and the oversaturation of CaCO3 in the sea water. The size and shape of Pd I are generally variable among the species within one and the same genus but considerably stable in each cavernicolous species. The coefficient of variation (100 × standard deviation/mean) for the maximum diameter of Pd I rarely exceeds 10. As emphasized in our preliminary study (Kase and Hayami, 1992), the size of Pd I is unusually large in many cave species, and Pd II are observed in relatively few species. Among 46 cavernicolous bivalves, the presence of Pd II is recognized in only 14 species. If the above-mentioned criteria are applied to this case, the present bivalves consist of 14 planktotrophic (30 %), 20 lecithotrophic (43 %), and 12 directly developing (26 %) species (Tables 4 and 5). If several doubtfully cryptic species are excluded, the ratio of non-planktotrophic species becomes still higher. Although the developmental types of western Pacific shallow-water bivalves have scarcely been analyzed, non-planktotrophic development is more frequent in the cave bivalves than in non-cryptic bivalves of the same region. In other words, the develop mental strategy of these cavernicolous bivalves is generally comparable with that of boreal or deep-water species. The shape of Pd I may also be meaningful. The cave species of the Philobryidae, except for Cosa kinjoi, show large and hat-shaped Pd I with a; central boss and a wide marginal brim, as usual for this family. Similarly, hat-shaped Pd I is known also in Bentharca decorata (Figures 44, 45), Chlamydella incubata (Figures 204, 210) and Limatida kinjoi (Figures 256, 259). The saucer-shaped summit of Pd I of Promicida insignis (Figures 20, 23) is also an abnormal feature in the Nuculacea. We presume that such a hat- or saucer-shaped Pd I suggests parental, care of larvae. In fact, we found several pre-released larvae within the living valves of Cosa waikikia, Dacrydwm zebra, Chlamydella incubata and C. tenuissirna. These facts lead us to suspect that parental care of larvae may occur in wider taxonomic groups than was ever thought (e.g. Arcidae, Propeamussiidae and Limidae), and that the size and shape of Pd I (and probably also developmental strategy) are considerably plastic even in one and the same genus (e.g. Bentharca, Chlamydella and Limatula).
8. Dominance of K-selectionThe diameter of Pd I in the cave bivalves is generally very large and often exceeds 10 % of adult shell size. This fact strongly suggests relatively small clutch size and low fecundity, Mainly on these grounds it was preliminarily inferred that .K-strategy predominates in the cave bivalves (Kase and Hayami, 1992). Some authors postulated that K-strategy also prevails in deep-water bivalves, chiefly because of their low fecundity and relatively stable physical condition (Calow, 1983; Mackie, 1984). Primary production by photosynthesis does not occur in lightless environments such as the deep-sea bottom and submarine caves. Suspension feeders in the caves are forced to share a small amount of nutrition which has been brought from exposed environments by a weak current. Although the carrying capacity must be very small in the caves, equilibrium populations, if anything, may be maintained like deep-water benthos. The resemblance of generic composition and reproductive strategy between the cavernicolous and deep-water bivalve faunas may be consistent with this assumption. Another important common factor between submarine caves and deep-sea bottoms may be the relative rarity of predators. According to the divers' underwater observations as well as collected remains, teleosts, asteroids, shell-breaking crustaceans and durophagous gastropods (e.g. naticids and muricids) are much less common in these caves than outside. Bored and injured-and-repaired shell -remains are less commonly met with. These lines of evidence strongly suggest a low-level of predation pressure in these submarine caves. In such an environment it,-is generally supposed that the biomass of suspension feeders approaches the carrying capacity. The constant deficiency of nutrition as well as the presumably saturated condition may force the cavernicolous bivalves to most efficiently utilize the limited food source of phytoplankton. K-strategy characterized by small clutch size and nonplanktotrophic development probably becomes more advantageous than wasteful &gemma;strategy characterized by large clutch size and planktotrophy. As generalized by Pianka (1970, 1978), however, &Kappa-selection may be commonly accompanied by slower growth, larger body size .and longer longevity of individuals. In a comprehensive discussion of the relation between phylogeny and ontogeny, Gould (1977) has attempted to test his hypothesis that progenesis and neoteny are commonly related to r-selection and K-selection, respectively. Little has been known about the growth rate and longevity of cave bivalves, but their stunting and progenetic (instead of neotenous) features are evident. Judging from the diminutive shell-size, the life cycle of these bivalves may be relatively short, even if their growth is slow. Is the γ-K model inapplicable to the strategy of cave bivalves? Or is our assumption of K- strategy for the cave bivalves inadequate? Jablonski and Lutz (1980) already pointed out that the relation between the body size (or mode of heterochrony) and γ-K spectrum in marine molluscs is not necessarily compatible with Pianka's (1970) generalization and Gould's (1977) prediction. We believe that nutrition deficiency (or small carrying capacity) is a decisive causal factor in the adaptive strategy of cave bivalves. The remarkable stunting and progenesis may be the only adoptable strategy against such a constantly oligotrophic condition. Large body size and retardation of sexual maturation would make it impossible to maintain the minimum population size necessary to survive. The amount of nutrition required by a large-sized individual would suffice for 1,000 individuals of a one-digit-smaller species, even if their longevity were the same. Progenesis as well as Κ-strategy must reduce the waste of nutrition. Among the cave bivalves, Malleus (Malmifimchis) sp., Pycmdonte tanigwhii and Glossocardia obesa are exceptionally large in body size, but they never inhabit the totally dark inner part of these caves. 9. Fossil records of the cavernicolous bivalvesThough the present cave bivalves generally exhibit archaic aspects, little has been known about their fossil records at the species level. This is probably because fossil bivalves of such diminutive size have been overlooked or are only rarely described. Are these bivalves archaic at the level of supraspecific taxa? It is not necessary to say that the geologic range of each higher taxon largely depends on the ranking in classification as well as on the incompleteness of fossil records. In Table 6 the first appearances of the cavernicolous bivalve genera and families are shown on the basis of Cox et al. (1969) and some subsequent emendations; the first appearances are 1 (?) in the Devonian, 3 in the Triassic, 4 in the Jurassic, 2 in the Cretaceous, 16 in the Tertiary, 2 in the Quaternary, and 5 not represented by fossils. These figures may give the impression that the constituent genera of the cave bivalves are not particularly archaic.
Most of the constituent families, however, first appeared in Paleozoic or Mesozoic times; that is, 1 in the Ordovician, 1 in the Silurian, 2 in the Devonian, 1 in the Carboniferous, 2 in the Permian, 3 in the Triassic, 4 in the Jurassic, 2 in the Cretaceous, and only 2 in the Tertiary. Thus, most cave bivalves belong to long-ranging families. This is consistent with the composition of coexistent gastropods which almost lacks neogastropods except for a few species of the Columbellidae and Turridae (Kase and Hayami, 1992). Knudsen (1970) surveyed the geologic ranges of abyssal bivalve genera and families. Although his discussion was based on somewhat older data, taxonomic archaism is also obscure in abyssal bivalves. The archaism of cavernicolous (probably also deepsea) bivalves, we believe, is more clearly shown in their life mode than in their taxonomic composition. The present bivalves are mostly composed of epibyssate or semiinfaunal species, while few deep-burrowers and cemented species have been found. In other words, the archaic and defenseless mode of life before "Mesozoic marine revolution" (Vermeij, 1977) appears to be retained in cave bivalves. 10. Origin and adaptation of cavernicolous bivalvesThe origin and adaptation of the extraneous cavernicolous bivalves may be an interesting problem. The occurrence of several species belonging to shallow-water genera (e.g. Brachidontes, Septifer, Malleus, Epicodakia, Coralliophaga, Irus and Hiatelld) may be explained as an extended habitat of ancestral species (or populations). In addition, an exceptionally large bivalve, Pycnodonte (Pycnodonte) tanigtichii, is regarded as a relic of Tethyan fauna along with Neritopsis radida. It is bradytelic and probably has survived for a long geologic period since the Miocene by adapting to cryptic environments (Hayami and Kase, 1992). As summarized before, there are many species belonging to deep-water genera (e. g. Huxleyia, Pronitcula, Bentharca, Bathyarca, Gratis, Dacrydiinn, Parvamiissium, Chlamydella, Divarilima, Limatula, Carditella, Kelliella and Halonympha). Although none of them seems to be identical with deep-water species, their deep-sea origin is very likely. Most species of these deep-water type genera indicate lecithotrophic or direct larval development, and their migration for long distances must be difficult in comparison with planktotrophic species. The populations of these species must at present be almost isolated between caves. Nevertheless, they are often distributed widely not only in various caves of the Ryukyu Islands but also in similarly sheltered environments of Bohol Island of the Philippines and Palau Islands (Table 2). The assemblages of bivalves are somewhat different between Ie Islet (Okinawa) and Shimoji-Irabu Islet (Miyako Islands). Okinawa and Miyako are separated by a deep sea called "Kerama Gap" which is wider than 300 km and deeper than 1,000 m (Map 1). The endemism of several species (e.g. Cosa nchimae, Parvamiisshim crypticum, Divanlima elegans, Carditella iejimensis and Halonympha asiatica restricted to Ie Islet, and Ummella convava and Carditella shimojiensis restricted to Shimoji-Irabu Islet) may be explained by this barrier. It is noted that these endemic species are non-planktotrophic.
The geographic distribution of Cosa waikikia, Chlamydella incubata and Chlamydella tenuissima, however, is incredibly extensive, if their parental care of juveniles is considered. The first species is distributed in Hawaii, Samoa (?) and many isolated islands of the western Pacific; the second and third species, in many distant areas of southern Japan. It is still a mystery how these bivalves can have extended their distribution. Though there is no positive evidence, we now hypothesize that these species could tolerate long pseudoplanktic lives. Because the individuals of these species easily attach themselves by byssus to various objects, fishes, large crustaceans and some floating materials probably transport them for long distances. Another unsolved problem is related to the occurrence of three deep-water genera, Dacrydmm, Kelliella and Halonympha, in the caves. The cave species of these genera are morphologically more closely related to northern Atlantic species than to any described species in the Indo-Pacific region. Dacrydiiim zebra, another brooding species, is very close to Dacrydium viviparum from the deep water of the northern Atlan tic. Kelliella japonica is undoubtedly a close ally to Kelliella Trillions, also from the northern Atlantic. Halonympha has been known almost exclusively from the deep water of the Atlantic. As pointed out by many authors (e.g. Knudsen, 1970), however, it must be noted that bivalves of abyssal depths do not show clear provinciality; many species have cosmopolitan distribution. If we consider the incomplete and biased research on deep-water organisms, it is possible that such "Atlantic" species will be found in the Lido-Pacific in the future. When and how did the bivalves of deep-water origin reach these sublittoral caves? The following discussion is no more than a speculation. Although the present submarine caves must have been drowned during a post-Glacial sea-level rise, the adaptation of these deep-water ancestors to such caves is not necessarily a Holocene event. It is more likely that since the Pleistocene (or earlier) these cavernicolous bivalves have been wandering similarly sheltered environments in shallow waters. As was discussed by Bouchet and Waren (1979), coastal upwelling may be able to transport the larvae (even non-planktotrophic) of deep-water bivalves within a few days. It is known that larvae of marine animals are generally more tolerant of changeable temperature and hydrostatic pressure than are the adults. If they accidentally settle in a sheltered environment, they may be able to exploit a new habitat. Rapid changes of body size and developmental strategy may occur in adaptation. |