CHAPTER VIII
Discussion
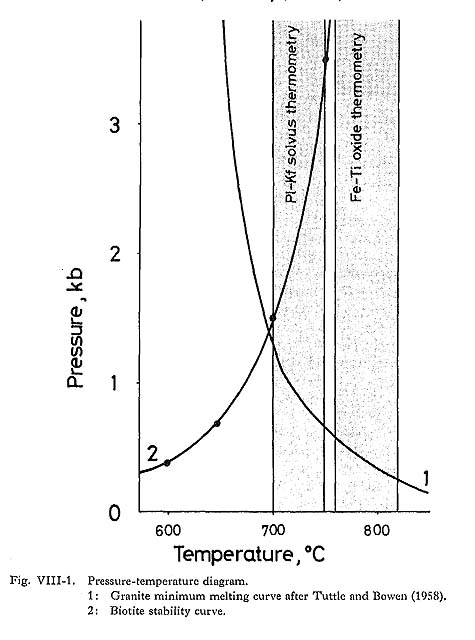
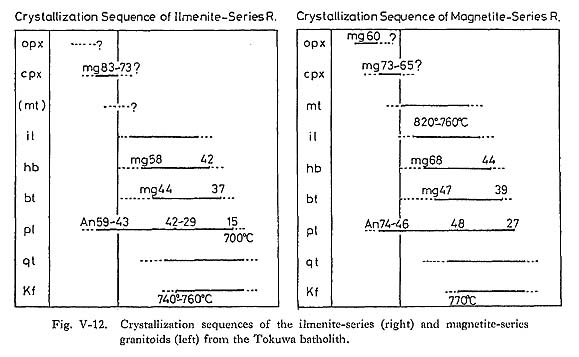
1) Intensive VariablesTemperature and Oxygen FugacityFeldspar equilibria provide an estimate of crystallization temperature. Takahashi (1980) determined the solubility of Or contents in plagioclase as a function of temperature in the pressure-independent range of 0 to 5 kbar: plagioclase from the batholithic rocks indicates equilibration temperatures of 770° to 700°C. Another method of temperature determina tion is the use of coexisting magnetite-ilmenite pairs (Buddington. and Lindsley, 1964). Fe-Ti oxide geothermometry yields magnetite-series equilibration temperatures of 820° to 760°C at oxygen fugacity of 10-14 to 10-15 atm (the equation by Itaya (1980) also gives 820° to 800°C and 10-15 to 10-16 atm, and that by Spencer and Lindsley (1981) gives 780° to 760°C and 10-15 atm) (Fig. VIII-1).
Pressure and H2O FugacityGeologic evidence and mineralogic features are used to estimate total pressure and H2O fugacity during magmatic crystallization. Because the presence of amphibole in the two series of granitoids means that the melt contained at least 3 wt % water (Burnham, 1979; Burnham and Ohmoto, 1980), the initial water content in the melt must have been about 3%. According to Wyllie (1971), the mineral sequence shown in Fig. V-12 might be expected to crystallize in a granodiorite magma (containing about 3% water) at a total pressure of about 2 kbar. The presence of miarolitic cavities and pegmatitic textures is commonly considered to indicate that the magma was saturated with a gaseous phase, at least at the time of consolidation (Burnham and Jahn, 1962). Assuming that H2O was the dominant species in the gaseous phase coexisting with the Tokuwa batholith magma and water saturation occurred during the latest stage, the intersection of the biotite stability curve with the "granite minimum" of Tuttle and Bowen (1958) in Fig. VIII-1 gives approximate values of H2O pressure and temperature of about 1.4 kbar and 690°C, respectively.
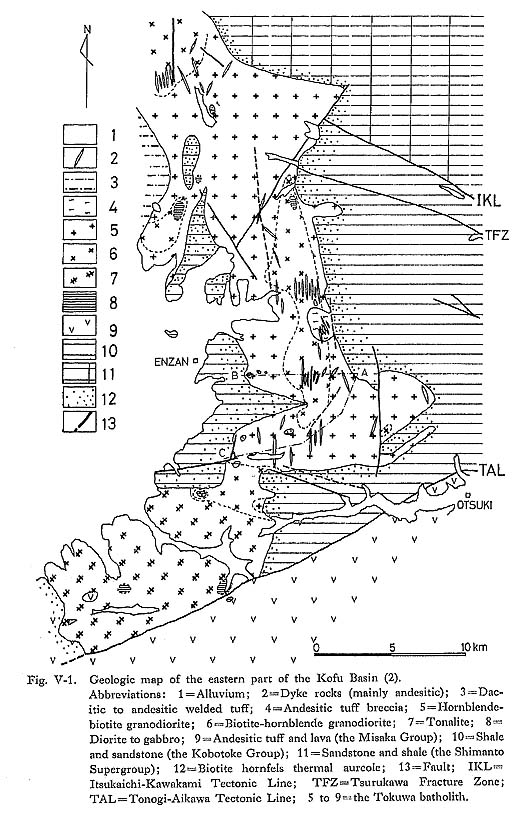
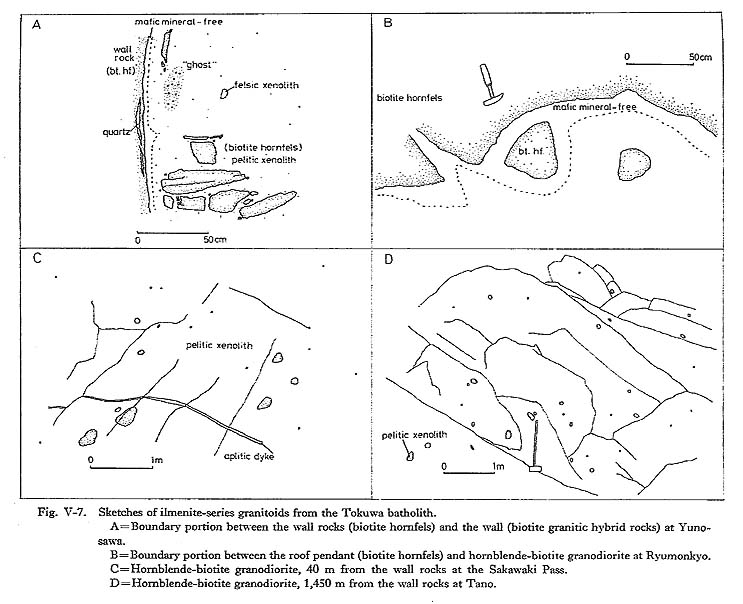
To summarize the data mentioned above, crystallization of the Tokuwa batholith rocks probably occurred under relatively shallow conditions. 2) Intrusion MechanismIt is generally considered that granitic magmas are emplaced in the upper crust by shouldering aside and updoming the country rocks ("diapir type") or by cauldron upheaval accompanied by stoping ("stoping type"). Granitoids formed through the former mechanism evidently intruded in the crust, and the latter are characterized by disharmonious, shallow-seated, or contact-aureole-type granitoid plutons (Walton, 1955; White et al., 1974). The country rocks around the Tokuwa batholith are commonly broken into blocks and pieces and have fallen into the batholith (Plate 13). The boundary between the granitoids and the country rocks is sharp and clean-cut along the steeply dipping wall contact. For instance, at Yunosawa (Fig. V-7-A), large blocks more than 20 m across and small frag ments of the country rocks are commonly included as xenoliths in ilmenite-series granitoids distributed in the marginal portion of the batholith. Most of the xenoliths can be assigned to country rocks near the present level, because they are identified as parts of adjacent country rocks. There is no evidence of mechanical deformation in the country rocks due to intrusion of the batholith. These field observations show that there was no forced ascension of magma to a shallow level and that magmatic stoping rather than diapirism or intrusion caused by squeezing of the magma was the major operative mechanism of emplacement of the batholith.
The granitic magma might have risen by forceful displacement in ductile rocks at greater depths, and when the magma reached the presently exposed level of the Kobotoke Group rocks, its continued ascent was probably achieved due to stoping of sediments adjacent to the magma. Tensile fractures and subsequently gravitational falling of xenoliths take place more easily in intrusion rather than in diapirism, because tensional strength is generally thought to be about one-tenth of compressional strength based on the theory of failure (T. Masuda, 1982, pers. comm.). The amount of xenoliths is too small to explain the volume of space occupied by the batholith, and most of the batholithic rocks have relatively low oxygen isotopic ratios (+7.4 to +8.6‰ for normal granitoids; +9.9‰ for hybrid rocks) and low initial 87Sr/86Sr ratios (0.7051, Shibata and Ishihara, 1979; 0.7040 to 0.7059, Shimizu and Yamana, 1983). The extent of digestion and assimilation of xenoliths or country rocks is hence thought not to be very large. Most of the xenoliths and country rocks once included in the underlying batholithic magma chamber are considered to have sunk to deeper parts of the present level. 3) Effect of Carbonaceous Matter in Sedimentary RocksOxygen fugacity as well as temperature and chemical composition seems to be an impor tant variable in the formation of the two series of granitoids in the Tokuwa batholith, particularly in the crystallization of the mafic minerals, including Fe-Ti oxide minerals, of the rocks. The bulk total Fe contents of the two series of granitoids arc almost the same at given SiO2 contents, but their ferric/ferrous ratios are markedly different, Fe3+/(Fe2++Fe3+) ratios of hornblende, biotite, and ilmenite in magnetite-series rocks are higher than those in ilmenite-series rocks (Table V-1). Therefore, it is concluded that magnetite-series gran itoids were created under higher oxygen fugacity conditions than were ilmenite-series granitoids. Considering the occurrence of ilmenite-series granitoids at the margin of the batholith, it is probable that reduction took place in the marginal parts of the batholith. H2O seems to be important in controlling oxygen fugacity in the igneous process (Wones and Eugster, 1965). H2O could act as an oxidizing agent and it would be important in the formation of magnetite-series granitoids.
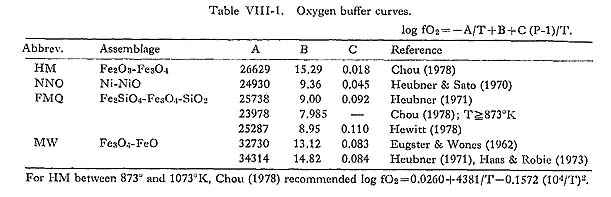
According to Sasaki and Ishihara (1979), δ34S (CDT) values of ilmenite-series granitoids in Japan (-11 to +1 ‰) strongly suggest that they were probably affected by light, bio-genic, sulfide sulfur derived from the crustal material. The role of graphite as a reducing agent has been reported by several workers (e.g., Miyashiro, 1964; Flood and Shaw, 1975; Kocis et al., 1977). Because the addition of graphite or carbonaceous matter to granitic melts is the most plausible process known at present to produce reducing magma, the author treats it quantitatively. Figure VIII-2 shows the log fO2-temperature relationship of the buffer systems shown in Table VIII-1. The FMQ and MW buffer curves gives similar results. Broken curves show the following reactions at various fCO and fCO2 values.
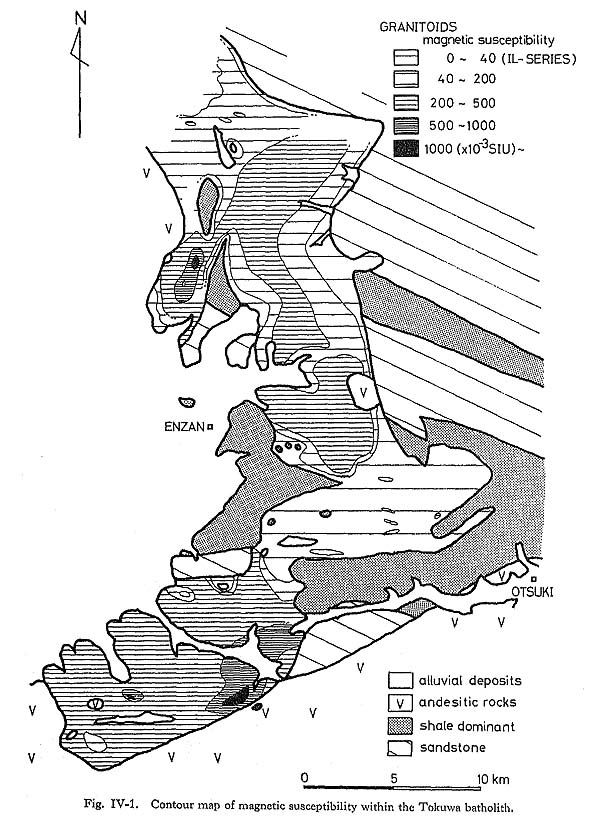
Short broken curves: [C] + 1/2 O2 = CO ……… (1) Points A and B in Fig. VIII-2 represent the fO2-temperature conditions of magnetite-series melt based on magnetite-ilmenite pairs obtained by Buddington and Lindsley's method (1964). The trend of magnetite-series melt is inferred to be the vector 4) Convection in the Tokuwa Batholith Magma based on Fluid DynamicsIf ilmenite-series granitoids at the margin of the batholith were formed by the assimi lation of sedimentary rocks, the mechanism of mass transfer must be considered in order to explain their distribution and occurrence. In general, there are two methods of mass transfer: diffusion and flow. Oishi et. al. (1975) determined the self-diffusion coefficients of oxygen (Doxygen) in "granitic melt" by a gas-liquids exchange technique using 18O as a tracer. The results are Doxygen=10-9.2 at 1000°C and Doxygen=10-10.6 at 900°C. From these data it is clear that 1-m transfer of oxygen in the magma requires about 0.5 Ma at 1000°C and 12 Ma at 900°C. Although several problems arise with this estimation (e.g., the change in D with the decrease of temperature and H2O contents) the minimum width of ilmenite-series granitoids, which is approximately 600 m (Fig. IV-1 and Type I in Table IV-1) cannot be explained solely by diffusion. Shaw (1974) also showed that the transport distances for mass transfer by diffusion in static magma are limited to a few m for most constituents and possibly a few tens of m for hydrogen.
Because the diffusion rate of elements in magma is very slow, it is necessary to discuss a few points concerning mass transfer by flow in order to consider the genesis of ilmenite-series granitoids. It is useful to infer the state of convection in the magma which might allow this mass transfer. The factors influencing whether or not convection will occur in a fluid in the presence of a vertical thermal gradient have been investigated intensively by workers in the field of fluid dynamics. The temperature gradient produces a density gradient and consequently a general tendency for a downward motion of the fluid near the center. Properties of flow and heat transfer appropriate to the fluid system have been worked out theoretically (Lighthill, 1953) and the theoretical predictions were experimentally verified to a remarkable degree (Martin and Cohen, 1954). Applications to problems of natural magmas have been discussed by several workers (e.g., Shaw, 1965, 1974; Bartlett 1969; Ewart et al., 1975; McCarthy and Fripp, 1980). The tendency of a magma body of a certain size to undergo convection of various types can be predicted on the basis of certain empirically derived dimensionless quantities, computed from certain intrinsic properties of the magma and from the dimensions of the chamber. The most important of these quantities is the Rayleigh number (Ra) for a cylin drical body, denned by the following expression:
(see Table VIII-2 for definition of terms and estimated values). The tendency toward convective heat transfer is strongly dependent upon the size and geometry of the chamber, and also on magma viscosity. Viscosity is important because of its immense variability depending on composition and temperature (Fujii, 1978).
Table VIII-3 shows the results of calculations of Rayleigh numbers using Eq. (1) for magma at various temperatures and water contents in magma chambers of various sizes. The temperature gradient (β) is arbitrarily assumed for the magma at its liquidus or above liquidus temperatures to be 4°C/km throughout, after McCarthy and Fripp (1980). When the value of 400°C/km is taken, however, the result is almost the same as that shown in Fig. VIII-2. The values for the thermal expansion coefficient (α) and thermal conductivity (k) used were from Shaw (1965) and the value of the specific heat of magma (Cp) from dark (1966).
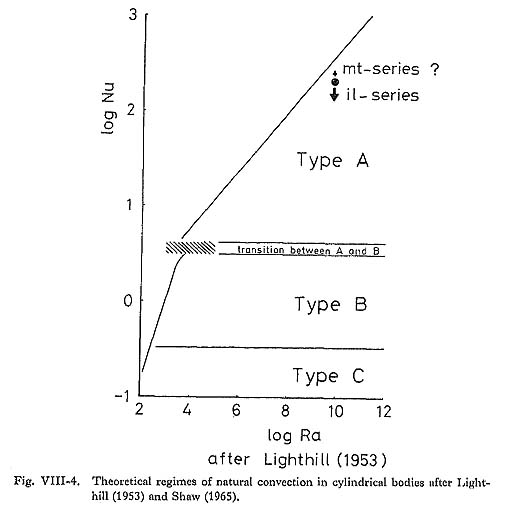
The average specific gravity of the magma (ρ) was calculated to be 2.4 g/cm3 by the method of Bottinga and Weill (1970). The pressure effect is considered to be negligible due to shallow conditions. The value (ca. 2.43) of magnetite-series is estimated to be slightly higher than that (ca. 2.41) of ilmenite-series. The magma viscosity (µ) was estimated to be 106 to 107 poise at 900°C and 105 to 106 poise 1000°C, for 2 to 4 wt % of H2O after Shaw (1965). It is assumed that the magma would behave as a Newtonian fluid at liquidus or above liquidus temperatures, such as 900°C, although the existence of gas bubbles and suspended crystals may lead to non Newtonian fluid behavior (Sparks et. al; 1977). Convection calculations have generally been used in two types of model. One is for the case of a horizontal sheet of magma, usually with constant temperature and water content and variable thickness, and the other is an extremely simple representation of cylindrical magma chambers crystallizing from the margins inward, with steadily decreasing temperature and increasing water content. The Rayleigh numbers here were calculated for the latter case of vertical tubes. The effect of change in viscosity with the increase of H2O content is not expected in the persistence of convection until a later stage of crystallization, despite the shrinking dimensions of the chambers. Theoretical calculations of critical Rayleigh numbers (Ra)crit for infinitely long tubes have been carried out (Yih, 1959; Verhoeven, 1968) and reviewed (Bartlett, 1969). Although (Ra)crit for onset of convection in finite vertical tubes is variable, it is not likely to exceed 1700. The Rayleigh numbers obtained for the magma under consideration are always greater than (Ra)crit for the onset of convection. The calculated values of Ra are indicative of convection (strictly speaking, type-A convection as will be stated later), Based on the critical Rayleigh number criterion, the Tokuwa batholith magma would have had convection currents. Figure VIII-3 demonstrates that natural convection occurs in granitic mag mas at typical viscosities at their liquidus or above liquidus temperatures except for small stocks. These results are consistent with those obtained by Shaw (1965), Bartlett (1965), and McCarthy and Fripp (1980).
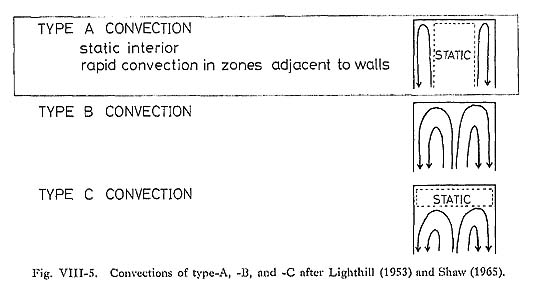
For the case of flow confined within a vertical cylindrical magma chamber, the relation ship between the Rayleigh number (Ra) and the dimensionless heat transfer coefficient, which is the Nusselt number (Nu) defined by the following equation, was studied: Nu = hL/k ……… (4) where h is the convection heat transfer coefficient, L the height of the magma chamber, and k the thermal conductivity of magma. Based on the Silveston correlation (Silveston, 1958), Bartlett (1969) reported an empirical correlation between Nu and Ra for the convection system below: Nu = ((Ra)/1700)1/3 ……… (5) This correlation enables us to distinguish three types of convection using the Ra alone (Fig. VIII-4). The boundary between type-A and type-B convection occurs at a Ra of about 105 and the boundary between type-B and type-C convection at about 103. Type-A convection involves a static interior with rapid convection in zones adjacent to the wall of the tube; type-B contains normal laminar convection filling the tube; and type-C is a weak convection in the lower part of the tube with static liquid in the upper part, as shown in Fig. VIII-5, The transition region implies a discontinuous change from type-A and type-B regions, and this was experimentally observed by Martin and Cohen (1954) in terms of oscillations between the two types of flow.
The correlation between Nu and Ra also allows for calculation of the temperature distribution within a convecting fluid. T1 - T2 = where T1-T2 is the temperature difference from bottom (T1) to top (T2) and qw is heat flux at the wall and roof of the magma chamber. Combining and rearranging Eqs. (3) to (6), the result is given as follows:
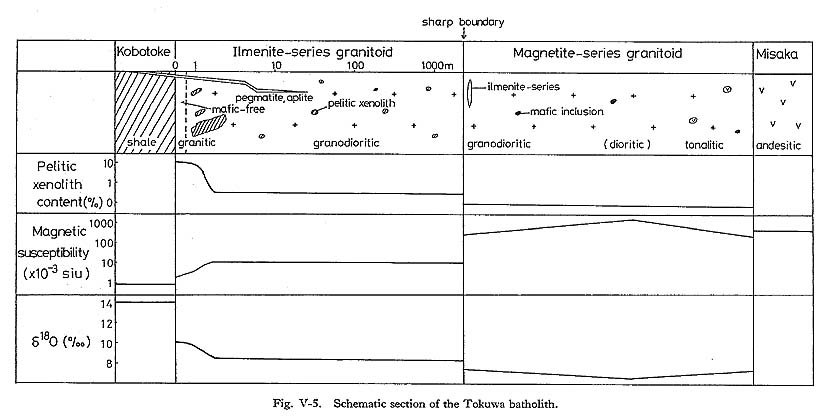
and assuming L/R=1, T1 - T2 = ( Taking into account that L = (T1-T2)/β ……… (9) is estimated to be 0.2 to 14 km.The velocity (U) of a fluid element during convection may be estimated from the expression U = K(Ra)2/3/L ……… (10) after McKenzie et al. (1974). Assumign Ra=6.7×109 to 6.7×1011, U may be estimated to be 2.5×10-2 to 0.5 cm/sec corresponding to 22 to 430 m/day. These values would be suffi cient to homogenize the convective part. 5) Formation Mechanism of Ilmenite-Series GranitoidsThe occurrence of ilmenite-series and magnetite-series granitoids in the Tokuwa batho-lith and their peculiar distribution were discussed in detail in Chapter IV. The distinctions between ilmenite-series and magnetite-series granitoids were recognized in the values of magnetic susceptibility, whole-rock chemistry such as Fe2O3/(FeO+Fe2O3) ratios and δ18O values, mineral chemistry such as mg values of hornblende, and the presence or absence of pelitic xenoliths. The boundary between the two series of granitoids is sharp. Drastic changes in magnetic susceptibility, contents of pelitic xenoliths and δ18O values at the boundary, and the homogeneity in ilmenite-series rocks are recognized (Fig. V-5). Geological survey shows that their distribution is correlated with country rocks: the dis tribution of ilmenite-series granitoids is correlated with that of pelitic country rocks, and pelitic xenoliths are commonly observed in ilmenite-series but not in magnetite-series granitoids. These facts indicate that there was "movement" in ilmenite-series melt, and that the "movement" would have promoted homogenization of the politic xenolith content and oxygen fugacity in the ilmenite-series melt. The "movement" is considered to have been convection based on the critical Rayleigh number criterion. It is recognized that predominant control of the formation of ilmenite-series granitoids of the Tokuwa batholith probably came from the dynamic behavior of the magma itself, particularly its tendency to convect. In this way, ilmenite-series melt would "monopolostically" have digested pelitic xenoliths. The boundary between the two series of granitoids may be considered to be a boundary between convective and static portions of the magma chamber. Because stoped pieces around which ilmenite-series granitoids generated, mainly by the diffusive mechanism, had lifted and stretched, ilmenite-series lenses in magnetite-series granitoids could occur (Plate 12). Some normative corundum-bearing magnetite-series granitoids are observed near the boundary (see Major Elements in Chapter VII), Convection is by far the dominant mechanism for transferring mass and heat, and promotes assimilation as a result. The convection may modify the original oxygen fugacity gradient in the melt through which the presence or absence of magnetite is controlled.
In the batholith adjacent to the Misaka Group rocks, this phenomenon occurred and formed magnetite-series granitoids containing igneous xenoliths. Effects of contamination and digestion of the igneous xenoliths were also recognized, for example, in the K2O/(K2O+ Na2O) ratios of granitoids adjacent to the Misaka Group rocks. Among several factors controlling crystallization of Fe-Ti oxide and mafic silicate min erals, oxygen fugacity seems to have been the most important one in the formation of the two series of granitoids in the Tokuwa batholith. The reducing agent, probably pelitic xenoliths derived from shale of the Kobotoke Group, was mechanically scattered in the portions close to the wall of the chamber. By this mechanism liquid state differentiation might have operated to produce significant compositional differences in the interior and the wall of the chamber. Through this independent convection system in the melt, homog-enization could be mechanically achieved (Fig. VIII-6).
The approximate 20% assimilation by sedimentary rocks, as suggested mainly by the oxygen isotopic compositions, is consistent with most major and minor element data (Table VIII-4). Shimizu and Yamana (1983) confirmed that the possibility of about 20% assimilation can be seen in the mixing curve defined by 87Sr/86Sr ratios and Sr concentra tions of the Tokuwa batholith and Kobotoke Group rocks. Sedimentary rocks naturally vary between extreme compositional limits, and thus their effects on. magmas tend to be conspicuous. Addition of a phase with which a liquid reacts may result in liberation of heat, whereas addition of a phase in which the liquid has not yet precipitated causes endo-thermic solution of both the added phase and, in some cases, crystals formed earlier (McBirney, 1979). In the first case, the proportion of liquid is reduced; in the second, it is increased. According to Pitcher and Berger (1972), pelitic sediments composed mainly of silica, alumina, and alkalis, corresponding to felsic magmas, tend to be more easily digested than quartzite or carbonate rocks. Compositional differences between the felsic melt at sufficiently high temperature and shale xenoliths aresmaller than the differences between the former and sandstone xenoliths, based on the mean values of shale and sand stone of the Kobotoke Group and granitoids of the Tokuwa batholith (Table VIII-4). Changes in felsic melt compositions may depend upon the degree of assimilation of felsic sedimentary rocks as well as magmatic differentiation. Consequently, sedimentary xenoliths are expected to have a tendency to extend the evolution of host magma by increasing the proportions of late differentiating liquids. The first stage of melting may produce a K-rich liquid, probably as a result of the initial breakdown of mica, and the composition of the melt could approach a granodioritic or granitic composition with increasing temperature.
As stated in Chapter IV, granitoids from the Tokuwa batholith have a strong correlation with the rock facies of country rocks in contact with the granitoids. The reason why ilmenite-series granitoids develop extensively near the contact with shale-dominant portion of country rocks (Fig. IV-1), can be considered systematically mainly based on the Rayleigh (Ra) and Nusselt numbers (Nu). An expanded convection width generally means a decrease in Nu values (=hL/k, see Table VIII-3), that is, type-A convection shifts toward type-B with a slight change in Nu as shown by the large arrow in Fig. VIII-4. Data on the thermal conductivity of shale and sandstone are insufficient and there are no data on it in the Kobotoke Group. The thermal conductivity of the Kobotoke Group shale may be assumed to be less than that of sandstone as shown in Table VIII-5. If so, the thermal difference from axis to margin (T1-T2) of the ilmenite-series melt in contact with country rocks of shale dominant portion can be estimated to be smaller than that in contact with sandstone dominant country rocks. The convective heat transfer coefficient, h=
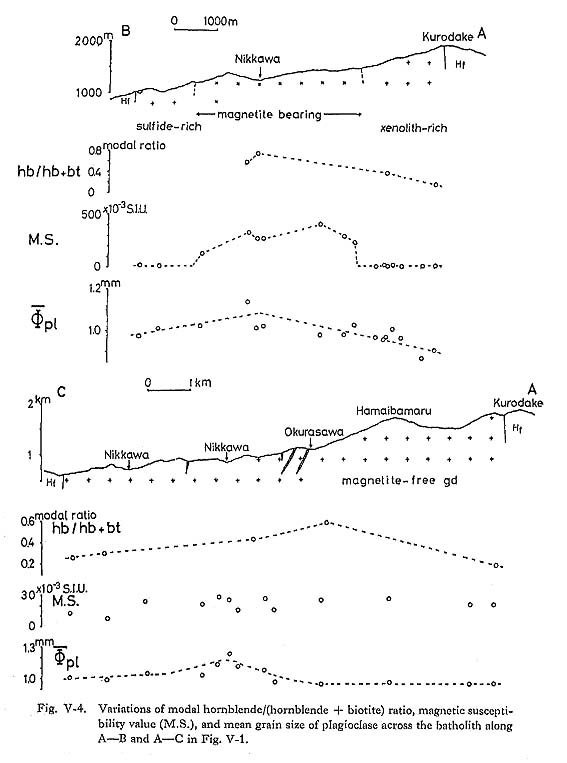
It is then easy to consider Ra (=R4αgβ/υK, see Table VIII-2) through Eq. (5). Thermal expansion coefficients (α) of I and II in Table VIII-4 are estimated to be 4.4 and 4.1, respectively, based on the method of Bottinga et al. (1983). These data do not include Al2O3, however, αI is expected to be larger than αII, because both mole fractions of Al2O3 are quite similar. Density (ρ) of the magma must be very important. Like many other properties, ρ of the liquid is strongly affected by chemical composition as well as temperature and pressure. ρI=2.38 and ρII=2.44 are calculated based on the method of Bottinga and Weill (1970). υI may be smaller than υII, considering that H2O decreases magma viscosity (Shaw, 1963). RI and RII, and KI and KII are respectively regarded as the same. Hence, βI < βII is plau sible due to the difference in thermal conductivity between shale and sandstone in order to reduce Ra. Once convection occurred in the batholith adjacent to sedimentary rocks, ilmenite-series melt could have developed as shown in Fig. VIII-6-I because ρilmenite-scrics < ρmagnetite-series. The ilmenite-series melt adjacent to the shale-dominant portion might have widened compared to that adjacent to the sandstone-dominant portion because ρI < ρII. Ilmenite-series-dominant portions in the batholith, shown in Fig. IV-1, might be the present surface A in Fig. VIII-6-II. Some of ilmenite-series rocks such Type II in Table IV-1 may be products of non-convection. Although a possibility still remains that ilmenite-series and magnetite-series granitoids were derived from separate magma pulses, probably involving different propor tions of source and interaction with crustal materials, the shape and distribution of the two series of granitoids and the variation of mean grain sizes of plagioclase across the batholith (Fig. V-4) exclude this possibility. Speed calculations of convection and xenolith sinking show that the flow of the convective magma exceeded the rate of sinking of the pelitic xenoliths enclosed in the magma. Under such circumstances the xenoliths may have been carried, and the magma flow was probably responsible for the disc-shaped pelitic xenoliths and their preferred orientation described in Chapter V.
|



 which exists between NNO and QFM buffer curves at around 800°C. The broken curves (1) and (2) take a position below vector
which exists between NNO and QFM buffer curves at around 800°C. The broken curves (1) and (2) take a position below vector 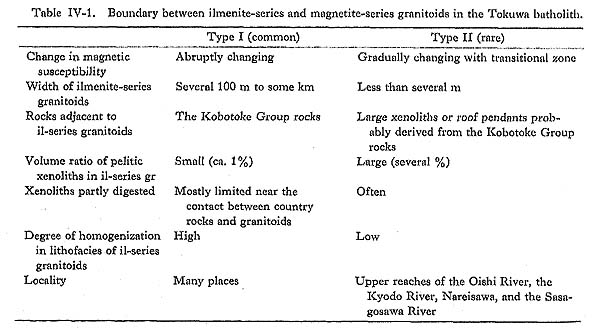
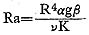 ……… (3)
……… (3)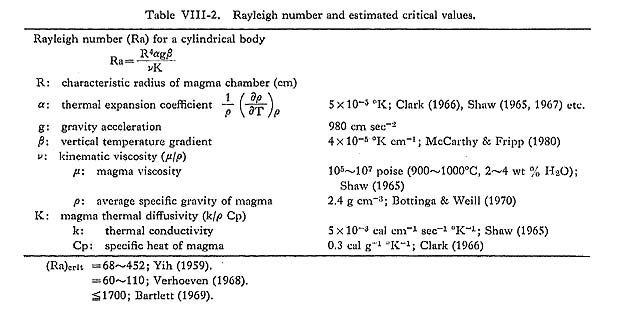


 w/h ……… (6)
w/h ……… (6)