CHAPTER VI
Mineralogy of the Tokuwa Batholith Rocks
6) Ilmenite
|
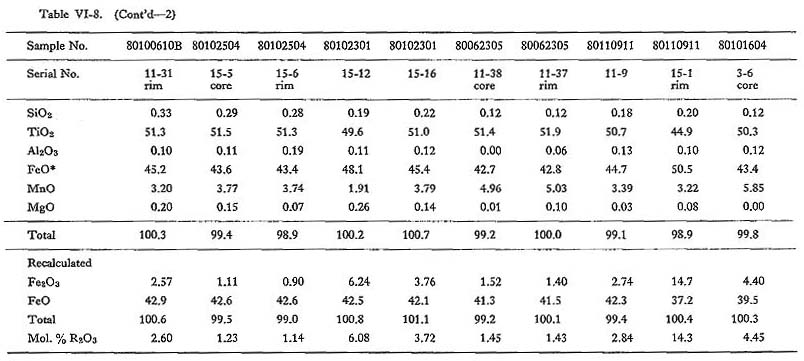
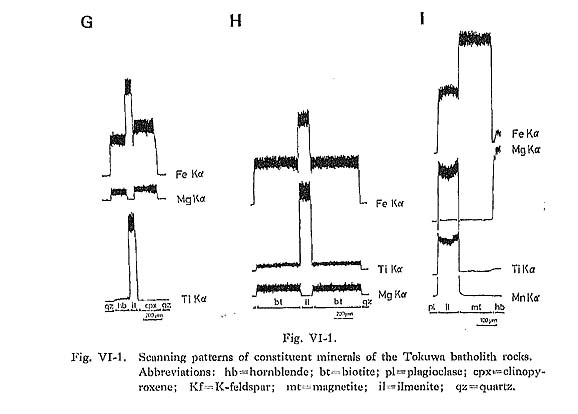
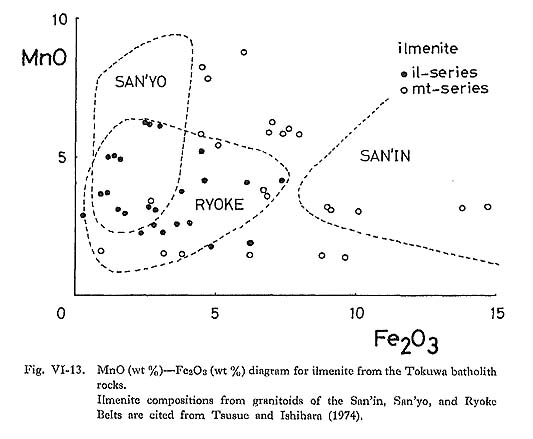
Ilmenite commonly occurs as a euhedral to subhedral crystal of up to 1 mm in size, and is usually included in hornblende and biotite. Ilmenite in magnetite-series granitoids rarely, occurs as lamellae in magnetite grains. Replacement of ilmenite by sphene (Czamanske et al., 1977) has not been observed in the Tokuwa batholith rocks. Thirty-six representative chemical compositions of ilmenite samples are shown in Table VI-8. The Fe2O3 contents of ilmenite were estimated using the method proposed by Carmichael (1967); Fe2O3 occasionally shows weakly zonal patterns with a Ti- and Mnrich rim and a Fe-rich core (Figs. VI-1-G and -I). TiO2 contents of ilmenite in magnetiteseries rocks (44.9 to 51.8%) are lower than those of ilmenite in ilmenite-series rocks (48.7 to 53.1%). MnO contents of ilmenite in magnetite-series rocks (1.29 to 8.81%) are higher than in ilmenite-series rocks (1.77 to 6.25%), and Fe2O3 contents of ilmenite in magnetiteseries rocks (0.89 to 14.3%) are also higher than in ilmenite-series rocks (0.27 to 7.13%) (Fig. VI-13). The relatively high Fe2O3 contents of ilmenite in magnetite-series rocks in the Tokuwa batholith indicate that the ilmenite in these rocks crystallized under higher oxygen fugacity conditions than did that in ilmenite-series rocks. Buddington and Lindsley (1964) reported that Fe2O3 contents of ilmenite are positively correlated with the oxygen fugacity under which ilmenite crystallizes.
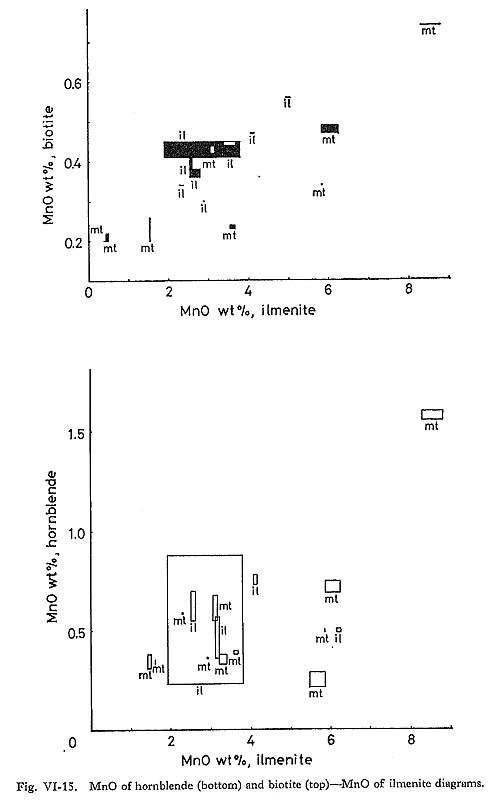
MnO contents of ilmenite are negatively correlated with FeO contents as calculated by the method of Carmichael (1967) (Fig. VI-14). Because Fe2+ is more easily oxidized than Mn2+, Fe2+ may be expelled from the ilmenite structure, and ilmenite in magnetite-series rocks may be rich in Mn. The difference of MnO contents of ilmenite in the mineral assemblages of magnetite-series rocks is not systematic. MnO contents of ilmenite lamellae in magnetite are nearly constant (5.86 to 5.96%) and MnO is often more abundant than in coexisting ilmenite. As for ilmenite in ilmenite-series rocks, the MnO contents of ilmenite (3.10 to 5.03%) included in biotite are higher than those (1.90 to 2.91%) in hornblende. MnO contents of ilmenite are more correlated with those of biotite than with those of hornblende (Fig. VI-15). These facts seem to indicate near re-equilibration between the ilmenite and the silicate minerals after crystallization, because limenite crystallized before hornblende and biotite.
The fact that Mn is more abundant in ilmenite as compared to that in magnetite has been well established on crystal chemical grounds (e.g., Buddhington, 1963; Neumann, 1974). Manganese partitioning between coexisting ilmenite and magnetite is governed by the fact that Mn2+ fits better into ilmenite than magnetite structures. The minerals from the Tokuwa batholith rocks satisfy this regularity. There is another problem concerning the Mn2+/Fe2+ ratio in ilmenite. This problem can be considered in the following sections. (1) Mn2+/Fe2+ ratio in the magma from which the mineral crystallized:
(2) Temperature of crystallization: (3) Oxygen fugacity in the magma: In summary, the degree of Mn enrichment is considered to increase with the increase in the Mn2+/Fe2+ ratio in magma at lower temperature and/or higher oxygen fugacity. Because ilmenite-series and magnetite-series melts had nearly similar solidus relations, as has been shown by feldspar thermometry, the Mn enrichment in ilmenite may be attributed to an increase in Mn2+/Fe2+ in melts at relatively higher oxygen fugacity. |