IV. MINERALOGY
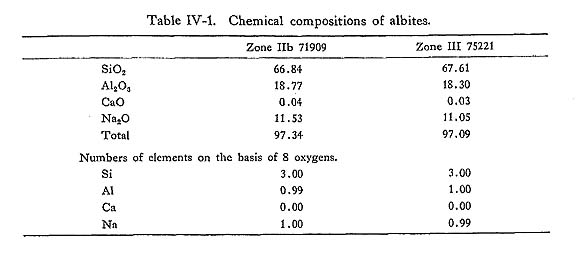
i) IntroductionThe basic, pelitic and psammitic rocks of this area are composed of chlorite, talc, muscovite, biotite, alkali amphiboles (magnesioriebeckite, subglaucophane, glaucophane), calciferous amphiboles (actinolite, barroisite), epidotes (epidote, piemontite), pumpellyite, prehnite, lawsonite, alkali pyroxenes (omphacite, aegi- rine-augite), pyralspite garnet, sphene, tourmaline, apatite, laumontite, carbonates (calcite, siderite), oxides (hematite, magnetite), sulfides (pyrite, sphalerite, cal- copyrite), sulfates, and graphite. In this chapter, the author describes the analytical data of minerals and discusses their stability. The chemical analyses of these minerals were carried out by the XMA microanalyzer Type 5A of J.E.O.L.. The compositional zoning of minerals were obtained by recording the intensity of characteristic X-ray by scanning the mineral specimen. Semi-quantative analyses were carried out using the empirical correlation factors obtained from the microprobe analyses of the studied minerals. ii) PlagloclasePlagioclase is albite which is homogeneous within the accuracy of probe analysis (Table IV-1). Albite in zones IIb and III is sometimes porphyroblastic and include epidote, sphene and quartz as inclusions, whereas in zones I and IIa it occurs in the matrix or as pseudomorphs after primary plagioclase.
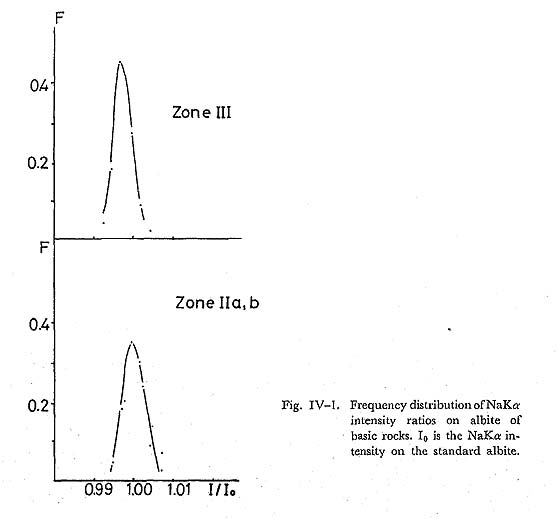
The composition of albite was determined using NaKα radiation. Fig. IV-1 showing the frequency distribution of albite composition indicates that the An content of albite slightly increases with increasing metamorphic grade. The An content of albite in the highest grade zone III is less than 2 molecular per cent.Similar observations have been reported from Franciscan (ERNST et al., 1970), New Zealand (BROWN, 1967), and other districts of the Sambagawa metamorphic ter rane.
iii) Amphibolesa) CalciferousamphibolesIntroductionMany authors discussed the chemistry of hornblende in the Ha'(Ca2Na2Mg3Al2Si6O22(OH)2) - Ts(Ca2Mg3Al4Si6O22(OH)2) - Ac(Ca2Mg5Si8O22(OH)2) - Gl(Na2Mg3Al2Si8O22(OH)2) tetrahedron in relation to the metamorphic grade. MIYA- SHIRO (1957), LEAKE (1968) and others concluded that Al in tetrahedral sites of amphiboles increases with increasing metamorphic grade in the low-pressure type regional metamorphic terranes. BANNO (1964) and BLACK (1973b) analysed am phibole of the glaucophane schist facies terranes and obtained the same tendency as that of low-pressure type metamorphic terranes. Recently, KLEIN (1969), BLACK (1973b) and TORIUMI (1974) analysed coexisting alkali amphibole and calciferous amphiboles and showed that the solubility of alkali amphibole component in calciferous amphiboles is sensitively dependent on temperature. OccurrenceActinolite is common in basic rocks of zones IIa, IIb and III, and occurs asso ciated with epidote, chlorite, albite, quartz, hematite and sphene. It also coexists sometimes with alkali amphibole in zones IIa and IIb, whereas in zone III it is associated with barroisitic amphibole. The mode of occurrences of actinolite is as follows. Zone IIa: In this zone, actinolite is very rare, its mode being less than l per cent. Very often, actinolite-alkali amphibole intergrowth grows epitaxially on relic augite, but independent actinolite in the matrix is not rare. Original basic rocks of this zone are alkaline and contain acmitic pyroxene, and this explains the common occurrence of actinolite-alkali amphibole intergrowth on augite. Zone IIb: In this zone, actinolite is a common constituent in the basic rocks. Alkali amphibole is not so frequent in this zone, probably because the original basic rocks are tholeiitic. Mode of occurrence of coexisting actinolite and alkali amphi bole will be described in detail in following chapter. Zone III: Actinolite is common in the basic rocks. Barroisitic amphibole is zoned with blue green core and colorless actinolite rim. In addition to epidote, chlorite, albite, and quartz, barroisitic amphibole often associates with actinolite. Actinolite is usually very fine grained (less 10 micron) while associating barroisitic amphibole is coarser-grained (30-50 microns) and forms individual grain. ChemistryFourty-two analyses of calciferous amphiboles were carried out. Some represen tative analyses are listed in Table IV-2. Analyses of paired actinolite and alkali amphibole are also listed in Table IV-8. This table shows that sum of Na and Ca in X sites of amphibole formula* ranges from 2.0 to 2.1.
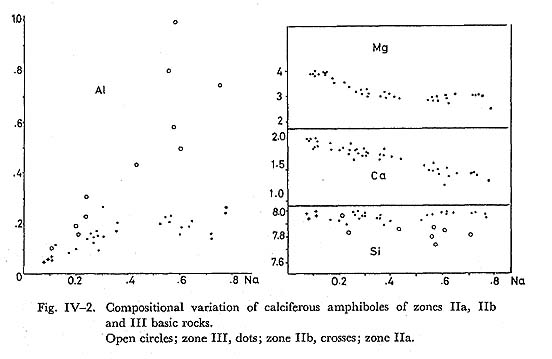
The substitution of elements is studied on the diagram showing the relationship between Na and other elements as indicated in Fig. IV-2. Two distinctive trends are recognized on Na versus Al, and Na vs Si diagrams, respectively. On the Na vs Al diagram, one group of amphiboles define the trend with Al/Na ratio of 0.3, and the other of 1.0. The former group corresponds to the high Si/Na ratio amphiboles on Na-Si diagram. As the amphiboles in question are approximatedly described as belonging to the Ac-Ts-Gl system, Al/Na ratio higher than 0.8 shows the presence of Ts-component. The Mg-content is rather constant and hence the amphiboles are approximately described by three component system as is done in Fig. IV-3. Clearly, the composition range in regard to Ts is larger in amphiboles for zone III basic rocks than those in zone IIb, and IIa. In zone III, actinolite in the matrix has similar composition to that forming rim around barroisitic amphibole.
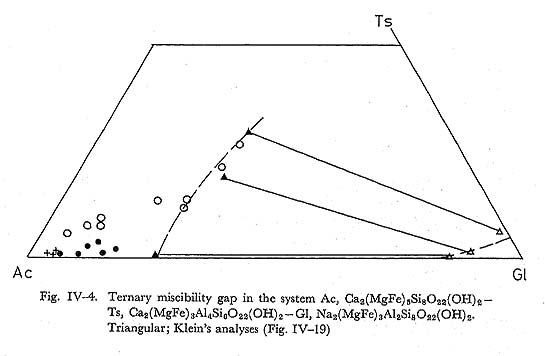
Zoning: Zoned amphiboles have scarcely been found in zone IIa, but in zones IIb and III they are common. The core has higher Na and Al than the rim, and this indicates that Gl-content in actinolite decreases from the core to the rim. The zon ing trends are shown in Fig. IV-3. DiscussionMiscibility gaps of hornblende, actinolite and glaucophane have been discussed by many authors. HIMMERBERG and PAPIKE (1968), COLEMAN and LEE (1967), KLEIN (1969), BLACK (1973b) and TORIUMI (1974) analysed paired actinolite and alkali amphibole, and actinolite and hornblende, in the high pressure type regional metamorphic terranes. Their results show the existence of miscibility gap. BLACK and TORIUMI established the temperature dependent solvus in the system of Ac-Gl-Ts and Ac-Gl-MRi, where MRi is magnesioriebeckite. According to their views, in the high grade zone of New Caledonia belt, calciferous amphibole coexisting with glaucophane falls in the compositional field ranging from Al-actinolite to barroisite. The available paired amphiboles belonging to Gl-Ts-Ac system are plotted in Fig. IV-4. The formation temperatures of these pairs are different, but the temperature dependence of ternary solvus can be infered if we could assume that the miscibility gap of the Cazadero district of the Franciscan metamorphic belt is same what as shown by dotted line. The compositional range of calciferous amphi bole from the epidote zone of New Caledonia and of zone III of the present area are also shown in the figure. The compositional range of these amphiboles are in harmony with the proposed miscibility gap, so far as the temperature of metamor phism decreases in the order of New Caledonian epidote zone, Cazadero, zone III, and zone II. The temperature of the paired amphiboles shown in the figure are based on oxygen-isotope thermometry data by EPSTEIN and TAYLOR (1967) and TAYLOR and COLEMAN (1968). On the other hand, Ts-content of calciferous am phiboles is limited by the following equilibrium relation; 6Cz + 3Am + 4Serp + 7Qz = 6Ts + 11Water, where Ts and Cz are in calciferous amphibole and epidote, respectively. Therefore, both Ts-content and Gl-content increase with rising temperature under constant PH2O in calciferous amphibole.
b) Alkali AmphiboleIntroductionAlkali amphibole is a common constituent in the basic rocks of the high pressure type regional metamorphic terranes. Chemistry and genesis of alkali amphibole have been discussed by MIYASHIRO (1957). MIYASHIRO and BANNO (1958) suggested that the stability field of magnesioriebeckite extends toward lower pressure than that of glaucophane in the glaucophane-magnesioricbeckite series,if alkali amphi bole coexists with chlorite and quartz. Recently, the chemistry of alkali amphibole was discussed in connection with the solvus between alkali amphibole and calciferous amphibole by KLEIN and TORIUMI. From their results, possible estimation is made on formation temperature of the metamorphism. OccurrenceAlkali amphiboles are common in basic rocks of zones IIa and IIb, but they have not been confirmed in the basic and pelitic rocks of zones I and III. Following assemblages containing alkali amphibole were observed.
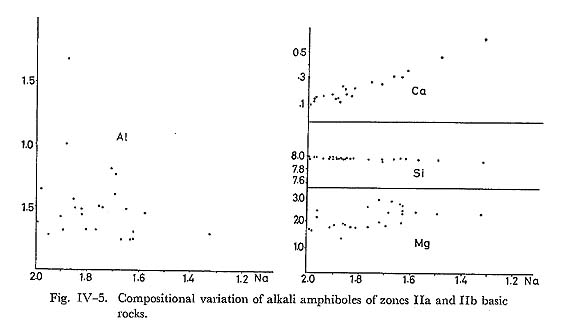
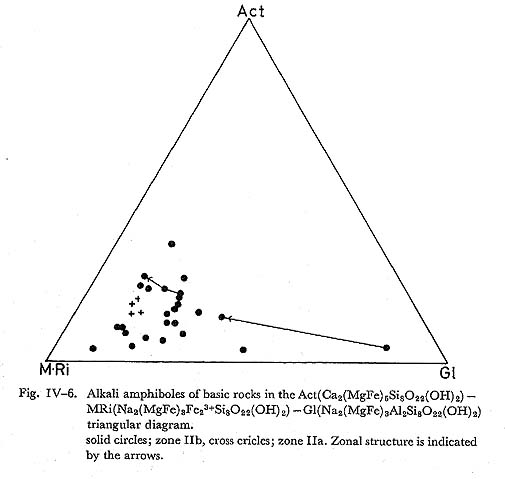
The assemblages 1. and 2. are in zone IIb, and 3. and 4. in zone IIa. The assemblages 1. and 3. are rather rare. The assemblage 1. occurs in hematite-rich rock and the alkali amphibole is subglaucophane or magnesioriebeckite. The assemblage 3. is confirmed in andesitic to dacitic compositions, and alkali amphibole is subglauco phane to magnesioriebeckite. The assemblage 2. represents the common alkali amphibole bearing basic rock, or glaucophane schist. In zone IIb, when basic rocks are schistose with conspicuous compositional band ing, alkali amphibole occurs not only around augite but also as individual grains in wholly recystallized layers. In the latter case, it usually occurs in quartz-albite layers and is rare in epidote-chlorite ones. In zone IIa, alkali amphibole, together with actinolite, grows on the (010) plane of relic titaniferous augite. Thus, the occurrence of alkali amphibole depends on the bulk chemistry of host rocks as described in the section of geology. ChemistryThe selected analyses of alkali amphiboles are listed in Table IV-3. The substitu tion of elements in alkali amphibole are examined on the diagram in which Al, Ca, Si and Mg contents are plotted against Na, as shown in Fig. IV-5. The figure shows that alkali amphiboles in this area are mainly magnesioriebeckite and subglauco phane, and glaucophane does occur, though rarely. A clear covariance of Na and Ca is recognized with fairly constant Mg and considerably constant Si. It follows that these amphiboles can be represented by the Ac-Gl-MRi triangular diagram as shown in Fig. IV-6. Clearly, actinolite contents in alkali amphiboles increase with rising temperature.
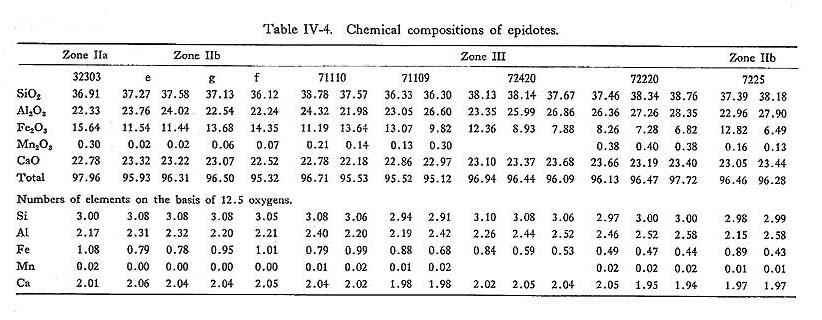
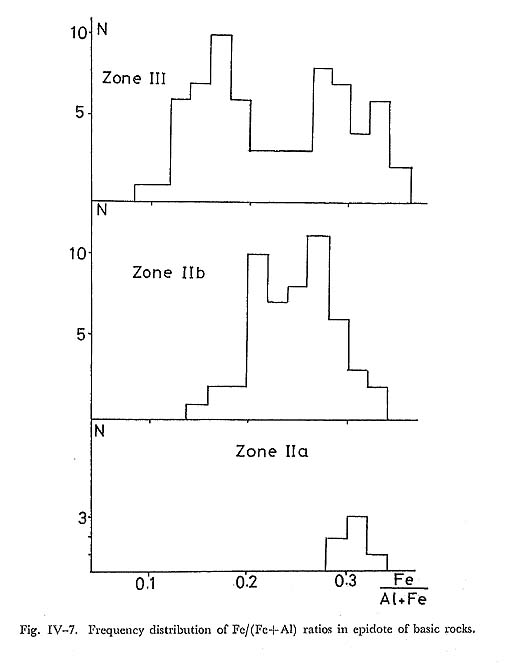
Zoning: Actinolite contents decrease from the core to the rim. Trends and ranges ofzoing are indicated on the Ac-Gl-MRi diagram as shown in Fig. IV-6. DiscussionIn zone IIa, alkali amphibole is usually magnesioriebeckitc and subglaucophane, while in zone IIb wide range of solid solution including glaucophane, subglauco phane and magnesioriebeckite occurs. However, amphiboles in zone IIa confined only around the vicinity of relic augite, and hence their compositions are strongly controled by local chemical environment of the nucleated grain. Thus the apparent trend of compositional range that it enlarges with increasing grade does reflect the role of physical conditions on the composition range of alkali amphibole solid solution, but it rather reflects the fact that the effective extension of chemical equilib rium enlarges with grade, there by making it possible to react albite and mafic minerals to form strict sense glaucophane. iv) EpidoteIntroductionEpidote occurs commonly in the basic, pelitic and psammitic rocks and it is one of major constituents in the basic rocks. MIYASHIRO and SEKI (1958) have pointed out the enlargement of compositional range of epidote in the Fe-Al-Mn epidote system with rising temperature. Recently, BROWN (1967) and ERNST et al. (1970) also reported the enrichment of clinozoisite component in epidote with increasing metamorphic grade in the Otago and Franciscan metamorphic belts, respectively. MIYASHIRO and SEKI have stated that the Al-enlargement of epidote is dueto the chemical reduction of rock accompanied with rising temperature, although BANNO (1964) prefered the composition based on the difference of bulk chemistry and various zones. OccurrenceIn zone I, epidote is seldom found in basic rocks, and it is uncommon in zone IIa. Epidote occurs in the matrix, and as pseudomorphs after primary plagioclase con sisting of albite, chlorite, quartz and epidote. In zone IIb, epidote is one of the common constituent minerals in the basic rocks. The intergrowth of amphibole and epidote is observed. Fine grained epidotes are often included in coarse-grained albite. Porphyroblastic epidotes containing actinolite, quartz and chlorite as in clusions are common in the basic rocks of zone III, and they show complex-zonal structure (TORIUMI, 1972). ChemistrySome of 48 analytical data of epidote from 10 localities are listed in Table IV-4. The Al/(Al+Fe) ratio ranges from 0.34 to 0.08, the frequency of composition of epidote is shown in Fig. IV-7. The diagram obtained from the partial analyses of Al and Fe indicate the enlargement of Al/(Al+Fe) ratio in epidote accompanied with increasing metamorphic grade. In zone IIa, epidotes are close to the pistacitic composition, and their compositional range is small. On the other hand, in zones IIb and III the compositional variation of epidote ranges from 0.15 to 0.33 and 0.08 to 0.33 in Fe/(Fe+Al) ratio, respectively.
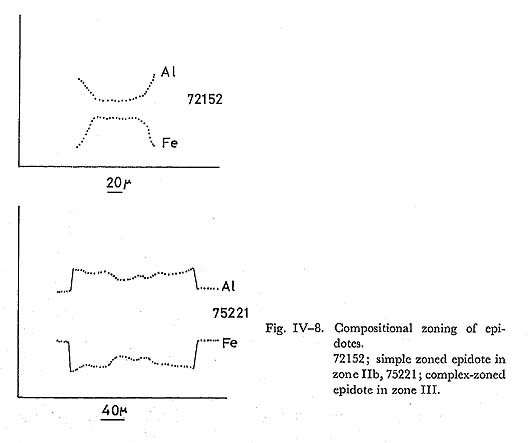
Zoning: The zoning of epidote in zones IIb and III was also described in the previous paper (TORIUMI, 1972). In that report, it has been noticed that there are complex-and simple-zoned epidotes. In the simple-zoned grain, Al-content grad ually increases toward the rim, whereas in the complex-zoned grain it gradually increases from center to outer part and the center and outer parts are surrounded by Fe-rich rim part (Fig. IV-8). Complex-zoned epidote appears only in the basic rocks of zone III, while simple-zoned epidote occurs in all zones. In zone II a, com positionaly homogeneous epidote close to pistacite composition forms aggregate of fine granular grains.
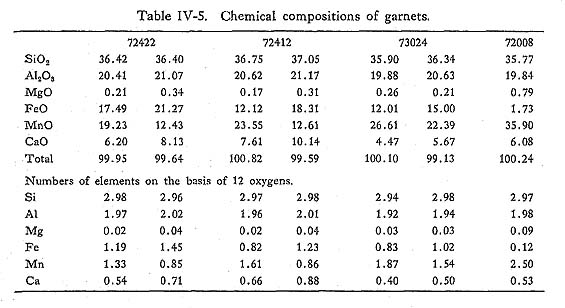
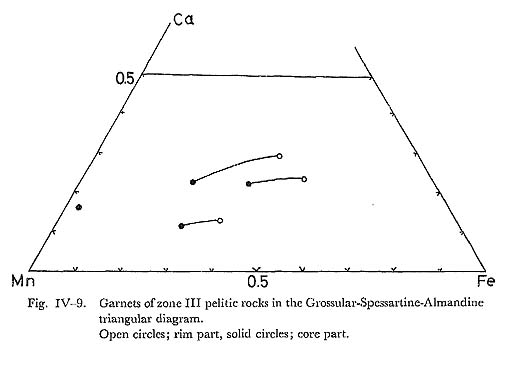
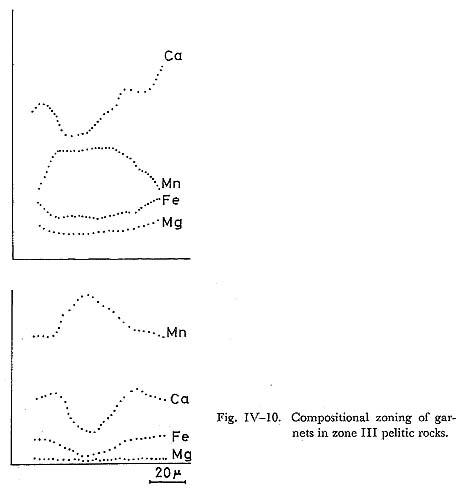
DiscussionContinuous zoning from 0.08 to 0.33 in Fe/(Fe+Al) ratio are common, and this conflicts with the solvus model proposed by STRENS (1965). Al-enrichment in epidote with increasing metamorphic grade was indicated by ERNST et al. (1970). It follows that the zonal structure in simple-zoned epidotes can be explained by rising temperature, and the rim of complex-zoned epidotes may be formed by decreasing temperature. It is also noticed that the range of compositional variation of zone IIb covers that of zone IIa, and the range of zone III also covers that of zone IIb and of zone IIa. Therefore, epidote crystallized under progressive metamorphism which means the upward trend of physico-chemical conditions from lower grade zone to higher grade zone. Further, in the rocks of zones higher in grade than zone IIa, pumpellyite becomes unstable, suggesting that the Al-enrichment of epidotes in zones IIb and III is derived from the decomposition of pumpellyite, as discussed in the previous chapter. v) GarnetIntroductionDecrease of garnet Mn-content in pelitic rocks with increasing temperature was observed by many authors, since MIYASHIRO (1956)'s conclusion in the Abukuma metamorphic belt. BROWN (1967), BANNO and KURATA (1972) and BLACK (1972a) and others analysed many specimens of garnet, and confirmed the MIYASHIRO'S suggestion. Theoretically, Mn-content is explained by the distribution of Mn and Fe between garnet and chlorite: when infinitesimal amount of garnet is produced from chlorite and quartz, Mn-content of garnet is controled by the distribution constant of MnO and FeO between them. With the conditions of the greenschist fades, this constant K denoted by BANNO (1965) is considered to be about 30, thereby showing the remarkably high concentration of Mn into garnet. Following these consideration, BANNO (1965) and others tried to explain the garnet zoning concearning Mn, Fe and Mg in progressive metamorphism. On the other hand, HOLLISTER (1966), ANDERSON and BURKLEY (1973) and many others tried to interprete the garnet zoning in terms of Rayleigh fractionation accompanied with surface equilibrium and FICK'S diffusion, respectively. Their simu lations were made on the assumptions of constant intensive variables different from progressive model. BANNO and KURATA (1972), however, proposed the growth model with rising T and/or decreasing fO2 or fH2O in the system of chlorite + garnet + epidote + quartz + fluid. In this section, the author intends to discuss the chemistry and genesis of zoning of garnet, and to examine the above mentioned three models of zoning. OccurrenceGarnet occurs restrictedly in the pelitic and psammitic rocks of zone III. The mineral assemblages participating garnet are as follows; 1) garnet + chlorite + muscovite + albite + quartz + sphene + graphite + rutile, Further, it is noticed that epidote-bearing assemblage without garnet is common in the pelitic and psammitic rocks of this zone. The variation of mineral assem blages does not appear to owe to the difference of bulk chemistry, because of existence of this variation in the same strata described in the previous sections. It follows that garnet may be produced by the reactants including epidote and chlo rite, as was discussed by BANNO and KURATA (1972). The rock with assemblage 2) consists sometimes of garnet + muscovite + chlorite, epidote + muscovite + chlorite, and quartz + albite layers. Combined with the absence of spatial hetero geneity of chemical composition of epidote, heterogeneous growth of garnet may be due to the local diversity of intensive variables except for temperature and pressure. Garnet producing reaction from epidote is as follows; 2Ca2Al2FeSi3O12(OH) = 2Ca2FeAl2Si3O12 + H2O + 1/2O2, Therefore, the effective intensive variables are T, P, fO2, and fH2O. Localization of fO2 and fH2O had been demonstrated by MUELLER (1961) and others. Garnet often includes fine grained hematite, quartz and sphene at the core. ChemistryEight analyses of garnet on 4 rock specimens were carried out as listed in Table IV-5. The table shows that the garnet in question are represented in the Mn-Ca-Fe garnets as plotted in Fig. IV-9. These garnets have composition similar to those of lower grade pelitic rocks of garnet zone in the glaucophanitic regional metamorphic terranes as studied by BANNO and KURATA (1972) and BLACK (1973a).
Zoning of garnet in respect to Ca, Mn and Fe is illustrated in Fig. IV-10, in which Fe-and Ca-contents show an increase from the core to the rim. Some of zoning profiles for Ca, however, indicate inflection point as shown in Fig. IV-10. Inflection point of Ca-zoning was described in detail by BANNO and KURATA. Profile of Mn-zoning appears to be a bell-shape named by HOLLISTER.
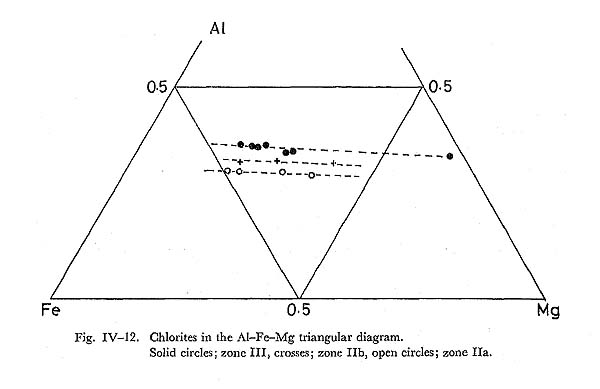
DiscussionFrom the petrographical consideration, it can be said that garnet was produced mainly by the expence of chlorite and quartz, being controlled by fH2O. These garnet occurs in the biotite zone, although in New Caledonia, pyralspite garnet appears commonly even in the lawsonite zone (BLACK, 1973a). Similarity of chemical composition of garnet and host rocks suggests that New Caledonia glau cophane schists are of higher T and P than those of the Sambagawa belt in a regional scale. The above mentioned garnet producing reaction is written as follows; Mn9Al6Si5O20(OH)16 + 4SiO2 = 3Mn3Al2Si3O12 + 6H2O Combining with the partitioning of Mn and Fe between chlorite and garnet, we obtain the reaction loop in the chlorite + garnet pseudobinary system, participating quartz and fluid. Therefore, as far as the system is in equilibrium under the same intensive variables, infinitesimal amount of garnet formed from this equation is unstable and must disappear. If temperature becomes higher, garnet becomes stable, and Mn-content decreases. These discussion were made by BANNO and KURATA. On the other hand, ANDERSOM and BURKLEY proposed the diffusion model for garnet zoning. Their diffusion model appears to be unreal by the following reasons: 1) Mn-content at the rim shows different value at different portion in a rock specimen, Therefore, we concluded that zoning, showing a decrease of Mn and Fe from the core to the rim, is due to rising temperature as is discussed by BANNO and KURATA. (vi) ChloriteIntroductionAlthough chlorite is the most common mineral in low grade metamorphic rocks, distinctive compositional variation with increasing metamorphic grade has not been clarified yet. SHIROZU (1960) and BANNO (1964) have pointed out the small range of the (MgFe)Si=AlAl substitution in chlorite. Further, ERNST et al (1970) has obtained the results that Si/(Si+Al) ratio, that is controlled by the above men tioned substitution, decreases with increasing metamorphic grade in the Shirataki district of the Sambagawa metamorphic terrane. OccurrenceChlorite occurs in the basic, pelitic and psammitic rocks of this area, as an inter stitial aggregate to other minerals. It participates in all assemblages listed in the section of petrography. In pelitic and psammitic rocks, chlorite occurs almost always as fine-grained aggregates intersitial to albite, quartz and muscovite. ChemistrySome of analytical data are given in Table IV-6. Ten analyses from basic rocks and six from pelitic rocks were carried out. Statistical examination of chlorite in regard to Al. Fe, and Mg were made (Fig, IV-11). Compositional difference of chlorites in some portions can. not be observed in a rock specimen. Analytical data are plotted in the Al-Fe-Mg diagram as shown in Fig. IV-12. It shows that the Alcontent falls in the remarkably narrow range in the same zone, and increases with increasing metamorphic grade. In spite of this tendency, Fe/(Fe+Mg) ratio is not related to the metamorphic grade, suggesting the subsidiary relation to bulk chemistry of host rocks. Al-content of pelitic chlorite coincides with that of basic chlorite in zone III.
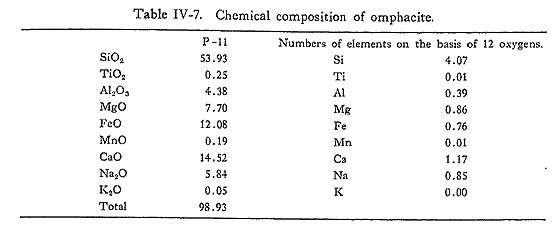
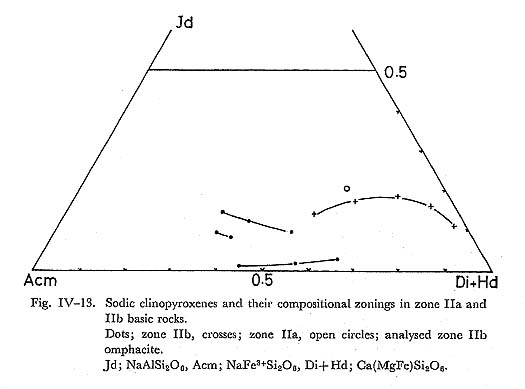
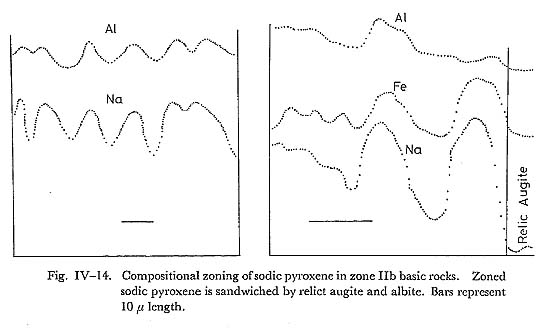
DiscussionSimilar to the result of ERNST et al. (1970), Al-enrichment of chlorite with increasing metamorphic grade is observed in this study. Combined with the wide range of Fe/(Fe+Mg) ratios in chlorite in a zone, this Al-enrichment appears not to be due to the bulk chemistry, but to the rising temperature. As is discussed in the previous section, chlorite is expensed following whole chemical reactions defining isograds, and its stoichiometry has low amesite component as compared with natural chlorite of zones I and IIa basic rocks. Hence, it may be concluded that the Al-enrichment is due to the rising temperature as mentioned above. vii) Alkali pyroxeneIntroductionAlkali clinopyroxenes in the glaucophanitic regional metamorphic terrane almost have the compositions among acmite, jadeite, diopside, and hedenbergite. The content of jadeite component in omphacite coexisting with albite and quartz has been determined experimentally by KUSHIRO (1969) at high pressure and temperature conditions, and it has been discussed thermodynamically by ESSEN and FYFE (1967) on the assumption of ideal mixture between jadeite and diopside, based on the jadeite-albite-quartz univariant equilibrium determined by BIRCH and LECOMTE (1960). On the other hand, CLARK and PAPIKE (1968) have found the P2 omphacite with the composition of Jd70Di30, and CHAMPNESS (1973) also found the antiphase domain submicroscopic texture in P2 omphacite by means of transmission electron microscopy. Therefore, ideal mixture approximation between jadeite and diopside is not appropriate. However, BELL and DAVIS (1969) have experimentally determined the critical unmixing temperature around 1700°K at 30 Kb and Jd50 Di50 composition. They also have determined the excess volume dependent on the content diopside content at 30 Kb as Vex = 2.65 X1X2, where X1 is the component fraction of jadeite in the mixture. If the excess free energy W denoted by Guggenheim is independent on temperature, the rate dTc/dP is about 15°C/Kb. Hence, Tc at 10 Kb can be obtained as 1130°C. Although, this critical temperature is not appropriate, because of the common occurrence of ordered omphacite with the composition of around Jd50Di50 in the eclogite from the glaucophanitic regional metamorphic terrane (Clark and PAPIKE, 1968). In the binary system of jadeite and diopside, the critical temperature of the order-disorder transformation dependent on the component fraction of diopside has not been determined experimentally, though Champness has suggested the existence of ordered P2 omphacite. Judging from the common occurrence of omphacite with intermediate composition between jadeite and diopside and existence of order-disorder transformation, BELL and DAVIS'S results may not be fitted in phase diagram of natural jadeite-diopside mixture. Occurrence and ChemistryIn the Onishi-Kagawara district, the jadeite content in omphacite is up to 0.3 component fraction, though SEKI (1960) has reported jadeite having the composttion of Jd80 coexisting with albite and quartz in the Nagatoro district east to the Onishi-Kagawara district. Omphacite in the Kanto Mountains almost occurs sandwiched by relict clinopyroxene and albite, coexisting with quartz in the basic rocks of zones IIa and IIb. Aggregates of omphacite without relict pyroxene are rare and they coexist with albite, quartz, chlorite, and phengite. Aggregated omphacite shows scarce zoning, and its chemical composition was analysed as indicated in Table IV-7. On the other hand, sandwiched alkali clinopyroxenes have distinct zonal structure as shown in Fig. IV-14. Semiquantative probe analyses of such sandwiched alkali pyroxene are shown in the Jd-Ac-(Di+Hd) triangular diagram. (Fig. IV-13).
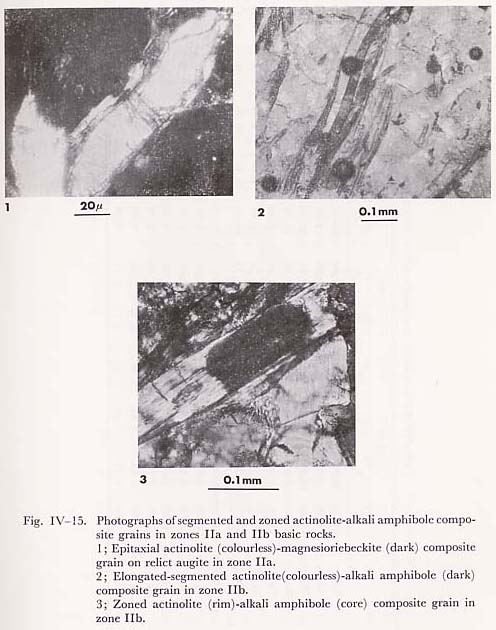
From the microprobe analyses, alkali clinopyroxenes in the basic rocks from the Kanto Mountains have the compositions of aegirine-augite varieties, of which the jadeite contents are up to 0.3 in the component fraction. From the Essen-Fyfe's approximation (ideal mixture of jadeite and diopside), pressure around 6 Kb can be obtained at 300°C and 0.3 fraction of the jadeite content in unzoned (aggregated) omphacite, coexisting with quartz and albite in the basic rocks of zone IIb. This pressure condition is available for the lawsonite-clinozoisite-quartz-albite, and glaucophane-epidote-chlorite-albite assemblages of the basic compositions, though the ideal approximation of jadeite-diopside mixture is not appropriate as discussed in the introduction. Compositional profiles in zoned sandwiched alkali clinopyroxene are shown in Fig. IV-14. This figure shows that the sandwiched alkali pyroxene is composed of aegirine-augite and augite grains. Hence, acmitic grains must have grown along the cracks and cleavages of host augite. In other words, the sandwiched alkali clinopyroxene domain is composed of fine grained reaction products and reactants (cpx). This conclusion is consistent with the zone IIb acmitic clinopyroxene grown along the cracks and cleavages of host augites in the basic rocks. The decomposition of clinopyroxene occurs preferentially in the deformed or cleavable clinopyroxene. viii) Subsolidus Phase Relation in Ts-Ac-Gl-MRi System at low Temperaturea) Actinolite and magnesioriebeckite systemCoexisting actinolite and magnesioriebeckite are common in basic rocks of zones IIa and IIb. Two types of association were recognized; zoned and segmented corn posite amphibole grains as described in detail in the previous section. In the former type, magnesioriebeckite invariably forms the core and actinolite the rim, and in the latter type, a composite grain consists of actinolite and alkali amphibole segments (Fig. IV-15). The analyses of coexisting actinolite and magnesioriebeckite were carried out at interfacies in segmented composite and zoned grains of zone IIa and zone IIb basic rocks. The segmented composite grain has the compositionally different interfacies even in one and the same grain (Fig. IV-16); in other words the compositional gaps are different from interface to interface in the composite grain. According to TORIUMI (1974), this fact can be explained by the idea that the segmented composite grain as a whole grew over a wide temperature range, but nucleation at different interface ceased at different temperature.
The compositional gaps in one segmented grain were represented on the FUJII (1967a)'s diagram
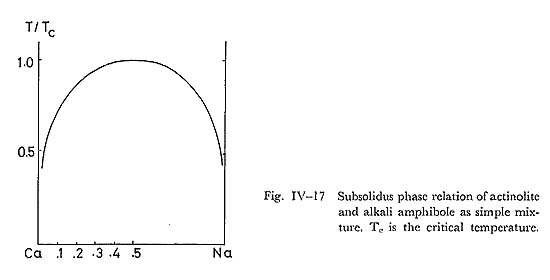
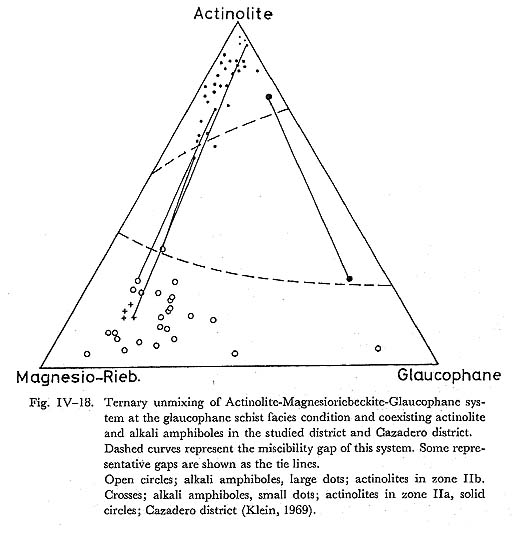
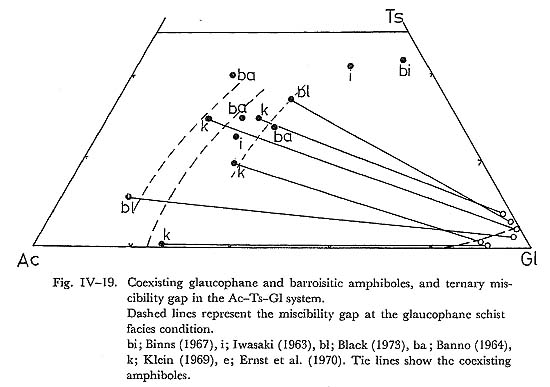
It follows that subsolidus phase relation of the actinolite and magnesioriebeckite system can be represented by simple mixture, with its critical ummixing at 330° combining the oxygen-isotope thermometry of the Franciscan glaucophane schist proposed by TAYLOR and EPSTEIN (1967). b) Actinolite and glaucophane systemCoexisting actinolite and glaucophane were analysed by LEE et al, (1966), COLEMAN and PAPIKE (1968), HIMMERBERG and PAPIKE (1968), KELIN (1969) and ERNST et al. (1970) in the Franciscan metamorphic belt. Some representative data of these amphibole pairs are plotted in Fig. IV-18. The analysed amphibole composite grains are composed of actinolite and glaucophane, and these textures are similar to the zoned composite grain of this area. The Mg/(Mg+Fe) ratio of these amphiboles ranges from 0.7 to 0.8. The compositional variation of analysed coexisting amphiboles is too small to estimate the critical ummixing points by means of FUJII'S diagram. Therefore, the miscibility gap of actinolite and glaucophane series is estimated only at such low temperature as the glaucophane schist fades condition as indicated in Fig. IV-18. This estimated compositional gap does not conflict with analytical data of paired amphiboles in this area. c) Glaucophane and barroisite systemKLEIN (1969) and BLACK (1973b) analysed coexisting actinolite and barroisite in the Tiburon penninsula, and New Caledonia, respectively, and their results are shown in Fig. IV-19. The ratio of Mg/(Mg+Fe) ranges from 0.7 to 0.8. The tie lines connecting barroisite with glaucophane show lines. The miscibility gap of the glaucophane and barroisite series can be infered by the data at the temperature of the glaucophane schist fades. Many analyses of barroisites reported by HASHIMOTO (1973), IWASAKI (1963), BANNO (1964), BINNS (1967) and others are also plotted on this triangular diagram, which suggests the consistency with the estimated miscibility gap between glaucophane and barroisite.
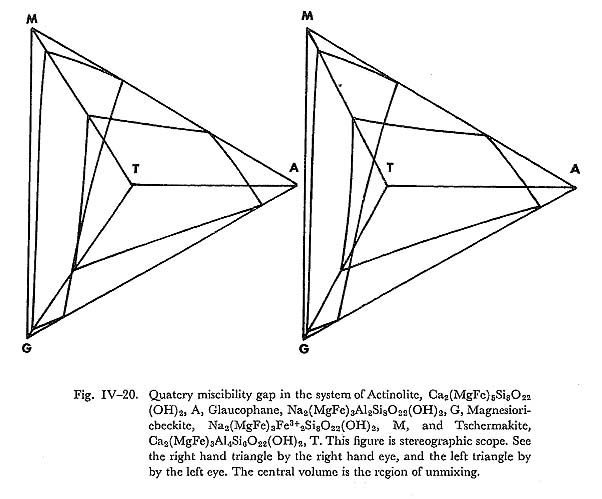
d) Glaucophaw and magnesioriebeckite systemThe continuous zoning from glaucophane to magnesioriebeckite occurs in zone IIb basic rocks of this area. Therefore, this series is continuous even at such low temperature as the glaucophane schist facies. This is in harmony with the conclusions of MIYASHIRO (1957). e) Miscibility gap of the Ac-Ts-Gl-MRi systemFrom the miscibility gaps of the barroisite-glaucophane and actinolite-glauco-phane system, we can estimate the miscibility gap in the actinolite-tscermakite-glaucophane system at low temperature. In Fig. IV-19 the possible two phase boundary is shown as dashed lines at the glaucophane schist facies condition. The miscibility gaps of glaucophane-actinolite and magnesioriebeckite systems may lead us to infer the ternary gap of actinolite-glaucophane-magnesioriebeckite system as shown in Fig. IV-18. Ternary gaps in the Ac-MRi-Gl and Ts-Ac-Gl systems do not conflict with analytical data of calciferous and alkali amphiboles in the low grade metamorphic rocks. On the other hand, from these two ternary gaps, the quartery miscibility gap of the system Ac-MRi-Gl-Ts may be estimated as shown in Fig. IV-20. This quatery gap is consistent with analysed amphibole data in the low grade metamorphic rocks. Therefore, the subsolidus phase relation of Ac-MRi-Gl-Ts system is such that the solid solution between Ts and Ac, which contains some amount of glaucophane and magnesioriebeckite components, is complete, and the series between Gl and MRi is also complete, while these two solid solutions do not make their mixture at such low temperature as the glaucophane schist facies condition.
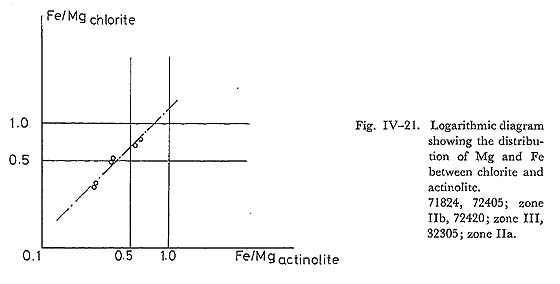
Note: The series between actinolite and hornblende has the miscibility gap as proposed by MIYASHIRO (1958), and thus that between actinolite and tschermakite may also has the miscibility gap at the low temperature. However, at the high pressure, amphibole of this series contains some amount of glaucophane component, and it is called as barroisite in this paper. ix) Solubility of Ts-content in Calciferous AmphiboleIn the glaucophanitic metamorphic terranes, barroisitic aluminous actinolite occurs widely coexisting with epidote, lawsonite, chlorite and quartz in basic rocks. In such terranes, barroisitic amphibole disappears, but common hornblende occurs in the high grade rocks, coexisting with quartz, chlorite and epidote. MIYASHIRO (1958) has pointed out the following hornblende producing reaction with increasing temperature; 6clinozoisite + (3serpentine + 4amesite) + 7quartz = 6tschermakite + 11water. (1) This equation controls the Ts-content in amphibole, if the Ts-component is soluble in amphibole at the temperature in question. Given the Cz-content in epidote and the composition of chlorite, the Ts-content in amphibole is governed by the equilibrium temperature. On the other hand, the deviation of equilibrium temperature and the Ts-content from equation (1) by the substitution of Fe for Mg may be small, judging from the fact that the partition of Mg and Fe between actinolite and chlorite is close to 1.0 (Fig. IV-21). Further, the range of Al/Si in chlorite is also narrow over the whole grades of regional metamorphic terrane. Therefore, we obtain the following equa tion derived from eq. (1); ln aTs - ln aCz = -4G°/6RT (2) This equation does not give the strict temperature-dependent XTs in amphibole, because of the unknown aTs for XTs. However, as Ts-content increase with rising metamorphic grade as discussed by BANNO (1964), BLACK (1973b) and in the previous section, and Cz-content in epidote is around 0.8 in high grade basic rocks, In XTs, continuously increases with increasing temperature. Therefore, the comparison of temperature condition of Kanto Mountains to other metamorphic terranes can be done by the Ts-content in amphibole coexisting with quartz, chlorite and epidote. The result of this comparison is such that the temperature increases in the order of Zone III of Kanto Mountains, Zone III of Kotu-Bizan (IWASAKI, 1963), Zone B of Cazadero (ERNST, 1965), Zone C of Bessi (BANNO, 1964) and epidote zone of New Caledonia (BLACK, 1973a, b), from the analytical data listed in Table IV-10. The oxygen-isotope thermometry by EPSTEIN and TAYLOR (1967), and TAYLOR and COLEMAN (1968) is 270-310°C in Cazadero, and 400-450°C in epidote zone of New Caledonia. These results, therefore, are consistent with the above mentioned order of temperature rise, estimated from the Ts-content in calciferous amphiboles.
|
|
* AX2Y5Z8O22(OH, F, Cl)2.[return to the text] |








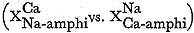 , and this diagram confirms that the miscibility gap of alkali amphibole and lactinoite solid solution series is symmetric. Therefore, it is concluded that solid solution is approximated by simple mixture (Fig. IV-17), having the constant excess Gibb's free energy. Zoned composite grains and many other segmented grains in zones IIa and IIb basic rocks were also analysed, and their analyses are plotted in Fig. IV-18. To avoid the confusion, the tie lines are not expressed in this figure. The compositional range of alkali amphi bole and actinolite in zone IIb covers those in zone IIa, thereby showing that the compositional gaps of zoned composite grains have the same sense as those of segmented grains.
, and this diagram confirms that the miscibility gap of alkali amphibole and lactinoite solid solution series is symmetric. Therefore, it is concluded that solid solution is approximated by simple mixture (Fig. IV-17), having the constant excess Gibb's free energy. Zoned composite grains and many other segmented grains in zones IIa and IIb basic rocks were also analysed, and their analyses are plotted in Fig. IV-18. To avoid the confusion, the tie lines are not expressed in this figure. The compositional range of alkali amphi bole and actinolite in zone IIb covers those in zone IIa, thereby showing that the compositional gaps of zoned composite grains have the same sense as those of segmented grains.